Everything You Need to Know About Isotretinoin

The Essential Info
What It Is: Isotretinoin, often known by its brand name Accutane®, is an oral medication approved for severe, scarring acne that is normally prescribed for 15-20 weeks. It is sometimes referred to as the “nuclear option” due to its ability to provide remission of acne in about 2/3 of people who take it, while at the same time causing concerning, potentially lifelong side effects, including severe birth defects.
Isotretinoin is a powerful drug that changes the skin and the body forever. Once you take it, there is no going back. As a real life example, because of the way isotretinoin can permanently alter eyesight, the U.S. military will not allow anyone who has previously taken isotretinoin to be a fighter pilot.
How It Works: It clears acne primarily through permanently reducing skin oil production, but also reduces bacteria, prevents clogged pores, and reduces inflammation.
Side Effects: Because it is an oral medication, it affects the entire body. Dry, cracked lips and skin are expected, and side effects such as nose bleeds, irritated eyes, rashes, fatigue, headaches, joint pain, and insomnia are just a few of the other common side effects. Some of these side effects may be lifelong, leaving the user with, for instance, a lifetime of impaired joints, or the need to use lip balm several times a day for the rest of their life.
Pregnancy: EXTREME CAUTION! ISOTRETINOIN CAUSES SEVERE BIRTH DEFECTS AND MISCARRIAGE. Females using isotretinoin must pass two (2) pregnancy tests before taking isotretinoin, and must use at least two (2) forms of birth control while on isotretinoin.
Suicide & Depression: Many people swear that they are more depressed or suicidal while taking isotretinoin, and while the research we have thus far does not show a connection, anyone taking isotretinoin should closely monitor their mental state.
Take with a High-fat Meal: For isotretinoin to get absorbed into the bloodstream, it should be taken with a high-fat meal (20 grams of fat or more).

The Science
- What Is Isotretinoin (Accutane)?
- How Does Isotretinoin Work?
- Side Effects
- Pregnancy
- Suicide and Depression
- Take with a High-fat Meal
- Do Not Buy Isotretinoin on the Internet!
- Presentation of Bias
What Is Isotretinoin (Accutane)?
Isotretinoin, originally known for its brand name Accutane®, or Roaccutane® in parts of the world, was discovered in 1979 when it was first given to patients with severe acne, most of whom reacted with dramatic and permanent clearing of their acne symptoms. It is a vitamin A derivative (13-cis-retinoic acid) that is administered orally in pill form with a meal that contains an adequate amount of fat,1 normally for 15-20 weeks (3.5 – 4.5 months),2 although it is also sometimes prescribed at lower dosages for up to 6 months or longer.
Isotretinoin was originally recommended for people with severe acne that did not respond to other treatments,3 but has gained in popularity in the past 25 years and is prescribed more frequently for less severe acne.4-6 This practice is controversial because isotretinoin is a systemic medication that affects every bodily system and may cause lifelong side effects.
Other medications are normally not prescribed at the same time, but in cases of extra-severe acne, physicians sometimes prescribe a few weeks of oral corticosteroids to quickly reduce inflammation before beginning a course of isotretinoin.7
How Does Isotretinoin Work?
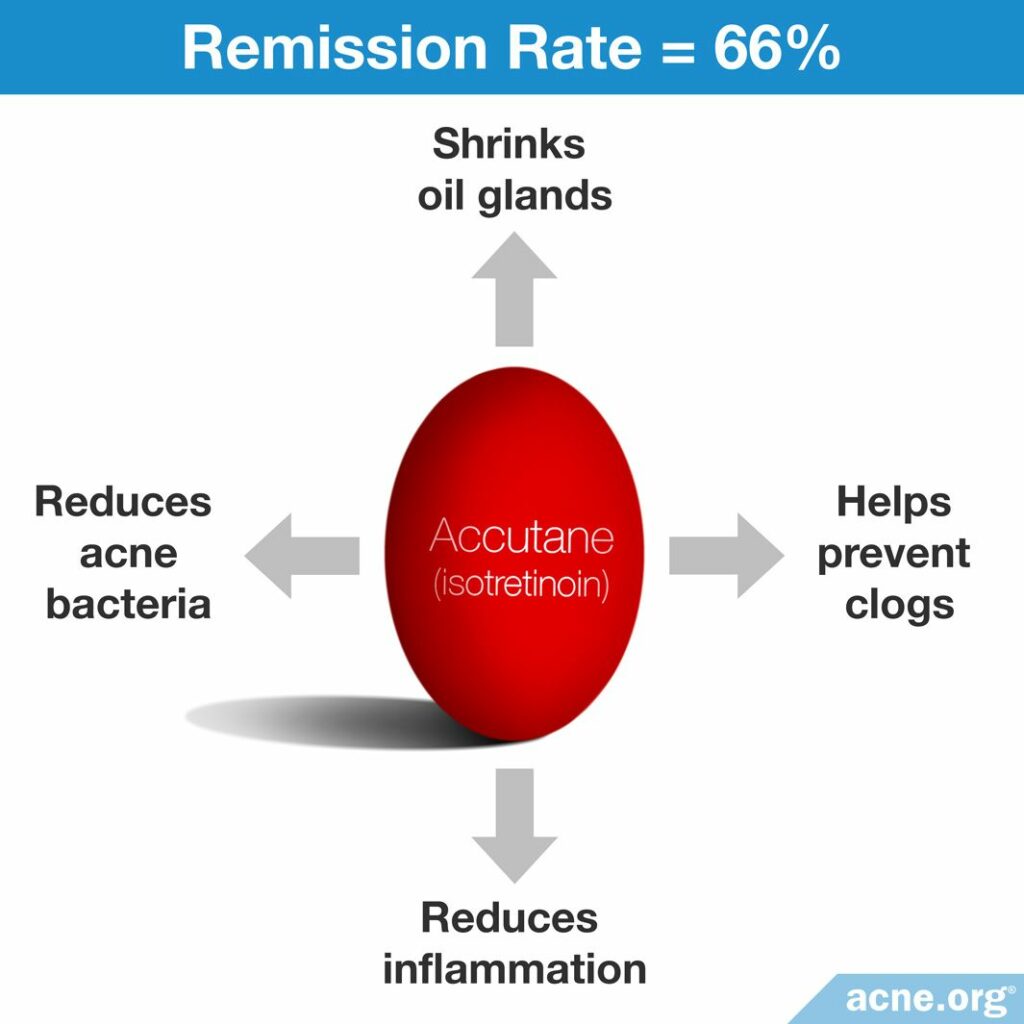
Exactly how isotretinoin works on a cellular level is unknown but we do know that it helps combat the four ways that acne develops.
- Reduces skin oil: It dramatically reduces the size of the skin’s oil glands (35% – 58%) and even more dramatically reduces the amount of oil these glands produce (around 80%).8-11
- Reduces bacteria: Acne bacteria (C. acnes) live in skin oil. Since oil is dramatically reduced, so is the amount of acne bacteria in the skin.9
- Prevents clogged pores: It slows down how fast the skin produces skin cells inside the pore, which helps pores from becoming clogged.11-12
- Reduces inflammation: It has anti-inflammatory properties.11-12
Initial worsening of acne: Acne may get worse within the first month for about 30% of patients, but after the first month results kick in and are usually dramatic.13
Efficacy: Isotretinoin works to achieve partial or complete clearance of acne in about 95% of people who complete a course, regardless of whether they have inflammatory or non-inflammatory acne.14 About 2/3 of people who take it experience long-term remission of acne symptoms.
Relapse: Studies show relapse rates between 14.6% to 52%, with a real-world average of about 1/3 of people experiencing a relapse. In these cases sometimes a second course is given.11,7,9,14-19 Patients who receive a cumulative dose of 100 – 120 mg/kg see the best results and lowest relapse rates. Patients who receive a lower dose relapse more frequently. Daily dosage depends on how much the patient weighs; 0.5 – 2 mg/kg is typical.1,15,17
Other factors that increase the chance of relapse are:
- Male gender
- Severe acne
- Not taking isotretinoin with an adequate amount of dietary fat
- Hormonal imbalances like polycystic ovary syndrome (PCOS) in women
Expand to read details on low-dose and intermittent-dose treatment
Low dosing: Traditionally, most doctors prescribe high doses of at least 1 mg/kg/day for relatively short periods of time (15 – 20 weeks). However, because many people develop severe side effects, more recently researchers started testing lower doses of isotretinoin administered over a longer period of time. Initial data is showing that patients with mild-to-moderate acne may be able to achieve long-term remission with significantly lower dosages, and thus suffer fewer side effects,20-22 including lower incidence of scarring. For people with more severe acne, staying on a lower dose of isotretinoin for a longer period of time until the full 120 mg/kg cumulative dose is achieved may be a way to produce long-term remission with significantly fewer side effects. The current recommendation based on this initial research is that the cumulative dose (the amount of isotretinoin that accumulates in the body) is the most important factor that determines treatment success.1 A good explanation on how to reach a desired cumulative dose of isotretinoin was published in the Journal of Clinical and Aesthetic Dermatology:

“If a patient weighs 70 kg, the cumulative total dose target of 120 mg/kg for his or her course of therapy would be 8400 mg (70 kg x 120 mg). Considering convenience and the practical consideration of capsule strengths, if the patient was started on 40 mg daily (slightly more than 0.5 mg/kg/day) for the first month, then increased to 60 mg daily (slightly less than 1 mg/kg/day) thereafter, it would take five months for the patient to reach the cumulative total dose of 8400 mg based on the 120 mg/kg target (1200 mg [40 mg x 30 days/month x 1 month] + 7200 mg [60 mg x 30 days/month x 4 months]). If the daily dose needs to be lowered due to side effects, the cumulative total dose target can be reached by lengthening the duration of therapy to what is needed to reach 120 to 150 mg/kg.”1
Intermittent dosing: Intermittent dosing (taking isotretinoin only one week of every month) also produces fewer side effects but may not work as well. It has been studied twice. In both studies people receiving an intermittent dose ended up receiving a lower cumulative dose, so we do not know if the poorer results are due to the intermittent administration of the drug or the lower cumulative dose. The first study compared an intermittent dose to a regular dose. Researchers gave patients in the intermittent dose group the same dose as patients in the regular dose group but for only one week out of the month. This resulted in only ¼ the cumulative dose after treatment ended. This produced slightly less clearing of acne and more than three times the relapse rate compared to the regular dose group. The second study compared a similar intermittent dose for only one week out of the month to a continuous low-dose every day. While exact numbers were not reported, from what we can glean from the study, this resulted in approximately 1/2 the cumulative dose for the intermittent group when compared to the continuous low dose group. Both groups in this second study achieved completely clear skin by the end of treatment, but more people relapsed in the intermittent group.21
Research on the optimal isotretinoin dosage is still ongoing. The authors of a 2018 systematic review published in the Cochrane Database of Systematic Reviews went through all available studies on isotretinoin:

The authors concluded that studies that tested different dosages and treatment schedules had inconsistent methods, making it difficult to compare across them to decide what dosage is best. They wrote, “Further trials are needed to evaluate different dose/regimens of oral isotretinoin in acne of all severities.”23
Side Effects
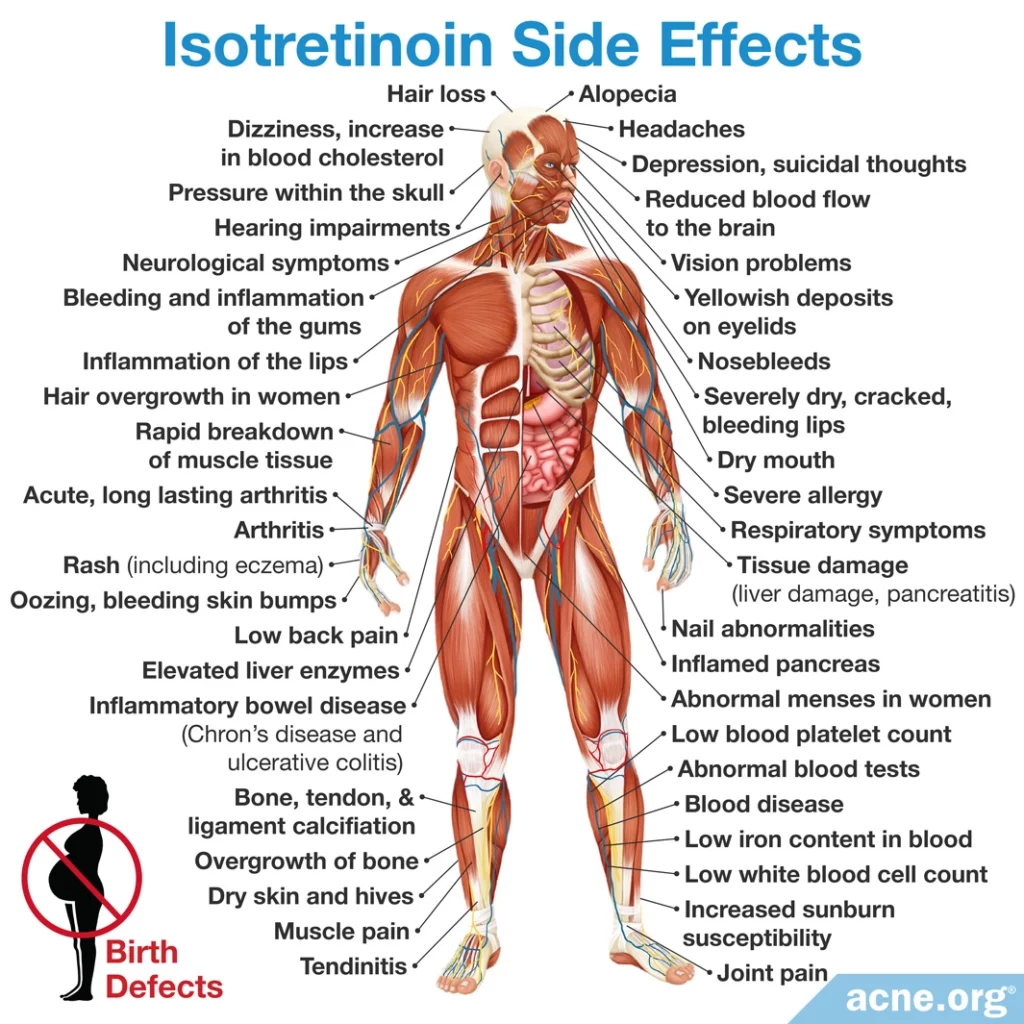
Isotretinoin is a systemic medication that affects the entire body. Side effects are numerous and widespread, and affect all patients.1-35
Side effects can be moderate and reversible, but in some cases can be severe or long-term.
Certain side effects such as severely dry skin/lips and nosebleeds are expected. Irritated eyes, rashes, fatigue, headaches, joint pain, and insomnia are also common.
Some people may experience more severe side effects elsewhere in the body, such as eye/vision problems, joint pain, psychiatric symptoms, blood imbalances, intestinal disorders, and many others. Unfortunately, some of these side effects can be lifelong.
Most important, and most catastrophic, is the likelihood of severe birth defects and miscarriage. Isotretinoin has the dubious distinction of being the #1 most birth-defect causing medication on the market.
Because isotretinoin is such a serious medication that comes with potentially severe and lifelong side effects, it is best to exhaust all other avenues of treatment first. At the same time, for patients with severe, deeply scarring acne, isotretinoin remains the most effective drug on the market.36 It can also be an option for females who suffer from severe acne due to polycystic ovary syndrome (PCOS) and who cannot take oral contraceptive pills, which are the standard treatment for PCOS-related acne.37 Some doctors believe that for patients with very severe acne, beginning isotretinoin treatment in spite of potential side effects may be preferable to waiting until the acne results in severe scarring, social anxiety, or depression.38
>> Click here for the full story on isotretinoin’s side effects
Pregnancy and Isotretinoin

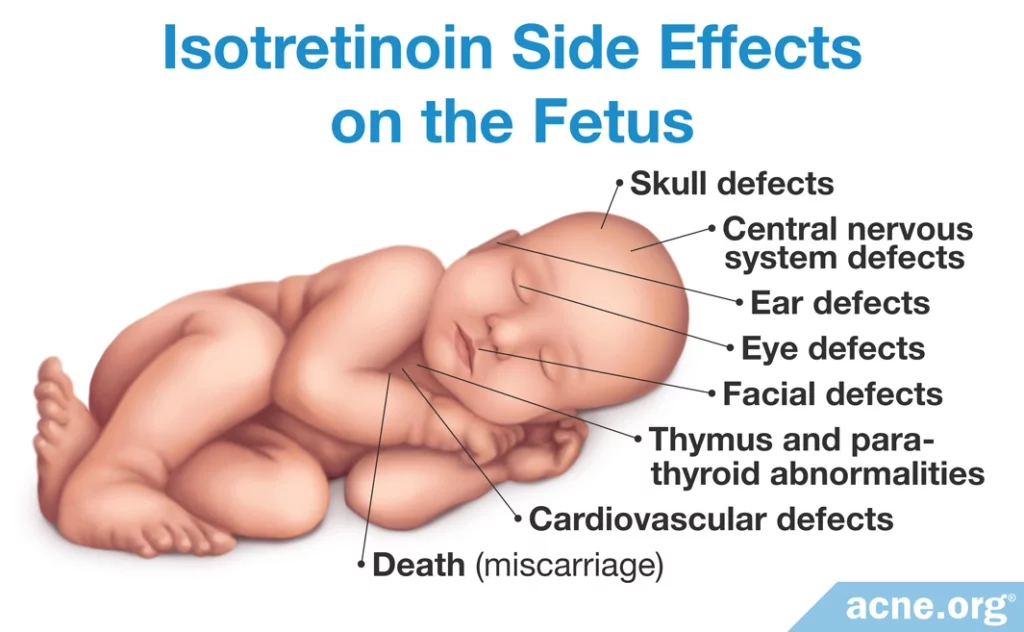
Isotretinoin is the #1 birth defect-causing medication on the market, with clinical research showing extremely high risk for birth defects if it is taken by pregnant women.1,3 Birth defects include:
- Skull
- Ear
- Eye
- Facial
- Central nervous system
- Cardiovascular
- Thymus and parathyroid abnormalities
- Miscarriage2
The risks are so extreme that the FDA approved the iPLEDGE program, which requires female patients of childbearing age to commit to using two (2) forms of birth control while on isotretinoin.4,5
iPLEDGE program telephone: 1-866-495-0654. iPLEDGE program website: ipledgeprogram.com
Expand to read more about pregnancy and isotretinoin
However, despite warnings to women not to get pregnant while using isotretinoin, studies published in Canada, the Netherlands, and the UK report a range of 11-24 per 1000 women getting pregnant while on isotretinoin.6-8 This is lower than the pregnancy rate in the general population of these countries which stands at approximately 50 per 1000 women, but is still unacceptably high, leading to tragic outcomes.
Another study performed in California looked at rates of pregnancy before and after the iPLEDGE program was implemented. Thankfully, they found lower rates of pregnancy among women using isotretinoin in California, but found that the iPLEDGE program had only modest results. Before iPLEDGE, 3.1 women in California per 1000 taking isotretinoin got pregnant, and after iPLEDGE this number dropped to 2.7. Researchers stated, “We found that most women on isotretinoin depend on contraceptive methods that require considerable adherence to be effective. Unfortunately, our results suggest that this degree of adherence is unrealistic for many women.”9 Abstinence, condoms, and the birth control pill were all cited as areas of non-adherence.
A recent study analyzed FDA reports on the iPLEDGE program and found that, while the program had reduced the number of unexpected pregnancies in women taking isotretinoin, there were still several hundred women getting pregnant on isotretinoin every year.10
The iPLEDGE program, while it helps to reduce pregnancies during isotretinoin treatment, may also create hurdles for patients that may disproportionately affect non-white patients. A recent study that looked at 418 acne patients taking isotretinoin in the U.S. found that 30.1% of white patients and 43.5% of non-white patients failed to complete their full course of isotretinoin and thus never reached the target cumulative dose. Many cited reasons such as computer problems or being unable to meet the requirements for lab tests and doctor’s appointments in the time windows required by iPLEDGE.11
The iPLEDGE program: Roche started with a program called SMART (System to Manage isotretinoin Related Teratogenicity) in 2000, which became the iPLEDGE program in March, 2006. Female patients of childbearing age are required to use two (2) forms of birth control while on isotretinoin.4,12
Suicide and Depression

Patients have reported depressive symptoms since the drug hit the market in 1982. Whether isotretinoin causes these depressive feelings remains a subject of intense debate. There are, after all, millions of people taking the drug, and there are bound to be people experiencing depression amongst them. Despite the confusion around this topic, Roche Pharmaceuticals, the makers of Accutane, added a warning to its label regarding suicide and depression in 1998.

An article published in the Cureus Journal of Medical Science in 2021 analyzed 9 recent studies that looked for a possible connection between isotretinoin treatment and mental health. The authors of the article summed up the latest research as follows:
- [X] No connection: 5 studies found no correlation at all between taking isotretinoin and mental health.
- [-] Isotretinoin may worsen mental health: 2 studies found that taking isotretinoin could cause mental health side effects such as depression, anxiety, or suicidal thoughts.
- [+] Isotretinoin may improve mental health: 2 studies found that taking isotretinoin could actually improve the mental health of patients with severe acne.1
Therefore, the preponderance of the evidence at this point is that isotretinoin does not appear to be linked with suicide and depression.1,21,25 However, to be safe, it is important for anyone taking isotretinoin to closely monitor their mental health while on the drug.2,5,26 Doctors prescribing isotretinoin can also check for signs of depression. A paper published in the Journal of the American Academy of Dermatology in 2016 recommends that all patients who are prescribed isotretinoin be screened for depression. The authors of the paper state, “Regardless of whether depression in these patients is rooted in the underlying acne or its treatment, the prevalence and serious nature of depression, suicide, and suicidal ideation demand attention. Given the chronic nature of acne treatment, dermatologists are uniquely situated to help screen for depression and suicidal ideation. It is our duty to care for all aspects of our patients’ health, including their mental health.”27
Expand to read the research on isotretinoin, suicide & depression
The question of whether isotretinoin causes psychiatric changes has been with us since it hit the market. However, media coverage on the topic spiked in 2000 when Michigan Congressman Bart Stupak’s son BJ committed suicide while on Accutane. Research began in earnest to determine whether there is a causal link between isotretinoin, suicide, and depression.
Quite a few studies have been conducted since. These have included large population-based cohort studies, retrospective analysis studies, relative risk estimates, prospective, observational, and longitudinal studies, and questionnaires performed in the United States and around the world.4-17 The first of these studies showed no conclusive evidence linking isotretinoin with depression or suicide.2,3 As the studies mounted, the data continued to show no evidence of a link.8-10,18 One study published in The New England Journal of Medicine found “431 cases of depression, suicidal ideation, suicide attempts, or suicide in U.S. patients treated with isotretinoin,” within a 10-year period. The article went on to note that the numbers listed do not exceed the U.S. suicide rate.19
If a researcher were to examine the evidence from 2000 until 2005, they would likely conclude that there is no evidence linking isotretinoin with suicide or depression.8-10 However, as is often the case, further analysis showed limitations to many of the studies.20,21 A general overview published in 2006 by the International Journal of Dermatology noted, “the overall lack of concrete scientific data limits any conclusion that can be drawn about a causal relationship between isotretinoin and psychiatric adverse events.”22
Then, in 2006, mice injected with the drug exhibited depression-related behavior. While animal studies often do not reflect human models, it was marginally intriguing.11 But even more provocative was a large cohort case-crossover study published in 2008 by the Journal of Clinical Psychiatry, which was the first controlled study to find a correlation between isotretinoin, suicide, and depression, albeit relatively minor.12 More recent studies have lent more credibility to the argument that isotretinoin does not negatively affect depression or mood,6,7 and several studies show significant improvements to depression, anxiety, and obsessive thoughts,13-17 presumably due to the power of isotretinoin to clear acne and thus increase quality of life.
A review of literature on the link between isotretinoin and depression in 2015 stated, “The major part of the dermatology community states that there is no causal link between isotretinoin and depression with this postulate: acne causes anxiety and depression; treating acne with isotretinoin is a way to manage depression.” However, there is still controversy, with critics pointing out the dermatology community’s tendency to not understand depression as well as the psychiatric community. “Literature studies have demonstrated two opposing views as to the role of isotretinoin from two differing clinical specialties. The psychiatric literature…suggests a causal link between isotretinoin and depression. The dermatological literature suggests that acne is an independent risk factor for depression and isotretinoin could be used to improve depression by treating acne and improving self-image. These differing views could be explained by a recruitment bias. Dermatologists may not have been aware of the occurrence of psychiatric disorders.”23
An even more recent meta-analysis published in the journal BMJ Open, which is a general medical journal read by both dermatologists and psychiatrists, found no connection between taking isotretinoin for acne and depression. On the contrary, the researchers found improved depression in patients after taking isotretinoin. Still, the authors of the meta-analysis wrote, “Future randomised controlled trials are needed to verify the present findings.”24
Take with a High-fat Meal

Isotretinoin is a fat-soluble molecule, meaning that it is best absorbed into the blood if taken with a meal that contains an adequate amount of fat.1-2 Based on the research thus far, it is prudent to ingest at least 20 grams of fat when taking a daily dose of isotretinoin.
Failure to take isotretinoin with fat-containing meals may account for some of the relapse that we see post-isotretinoin.
Expand to read research on isotretinoin and dietary fat
According to drug labeling information submitted to the U.S. National Library of Medicine by the makers of Accutane, “Both peak plasma concentration (Cmax) and the total exposure (AUC) of isotretinoin were more than doubled following a standardized high-fat meal when compared with Accutane given under fasted conditions. Therefore, Accutane capsules should always be taken with food. Failure to take Accutane with food will significantly decrease absorption.”3
So what kind of meal should be eaten when taking isotretinoin, and how much dietary fat should it contain? So far only 2 studies have been performed. The first asked participants to ingest approximately 20 g of fat (2 poached eggs, toast with margarine, plus 8 oz. of skimmed milk), and found that this was enough to approximately double the absorption of isotretinoin.2 The second asked participants to ingest 50 g of fat (1 bagel, 2 tablespoons of peanut butter, 5 slices of bacon, 6 oz. of apple juice, and 1 donut), and found that this was also enough to approximately double the absorption rate.4 Further research is required to determine exactly how much fat one must optimally ingest to reach maximum isotretinoin levels in the blood, but suffice it to say that isotretinoin must be taken with a meal which contains dietary fat to deliver its full potential.
Exceptions – Absorica® and Absorica LD™
Absorica®: In 2012, the FDA approved a new version of isotretinoin, called isotretinoin-Lidose, for sale in the United States that is marketed under the brand name Absorica. This formulation encapsulates isotretinoin in fat molecules and therefore reduces the need to take it with a fatty meal.1,4 The package insert states that Absorica “(1) is bioequivalent with Accutane when both are taken with a high fat meal; (2) has 83% greater absorption than Accutane under fasted conditions; (3) is not interchangeable with generic products of Accutane, and (4) can be dosed without regard to meals.” While data does show that absorption of Absorica is significantly greater than absorption of regular isotretinoin on an empty stomach, the claim that Absorica can be dosed without regard to meals may be skewed, since data show that even with Absorica, the amount of isotretinoin in the blood remains significantly higher when taken with a high-fat-containing meal.4
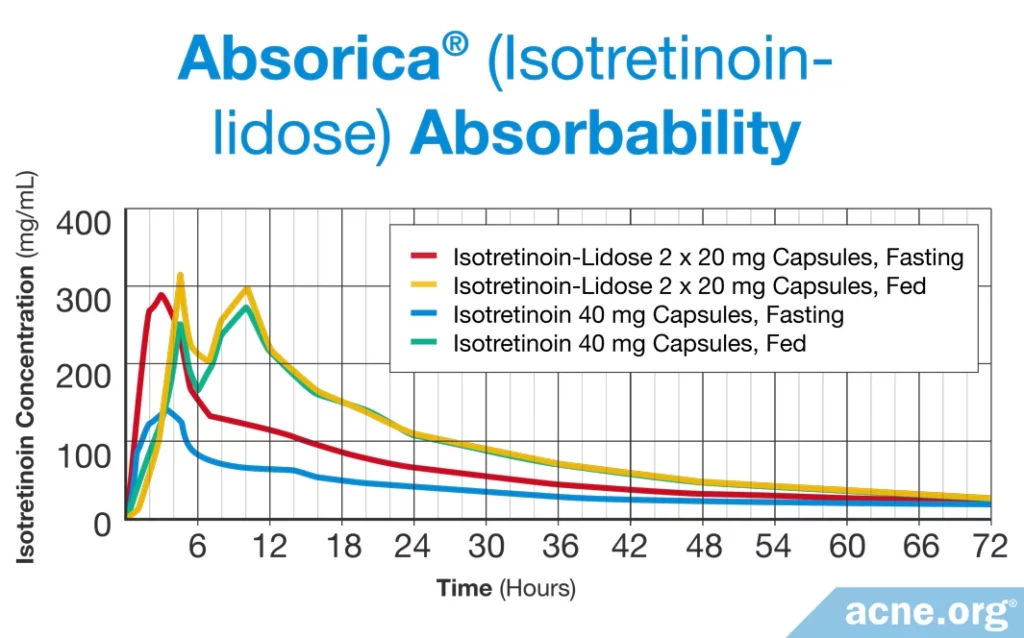
Thus, it may also be prudent to take Absorica with a meal which contains an adequate amount of dietary fat.
Absorica LDTM: To further improve the absorption of isotretinoin into the blood, the same company that produced Absorica developed a new version called Absorica LD.5 The FDA approved Absorica LD in 2019. Like the original Absorica, this drug contains isotretinoin encapsulated in fat molecules, but in this case, the isotretinoin is micronized, meaning it is split up into very tiny particles that are easier to absorb.
Two studies found that Absorica LD was absorbed twice as well as Absorica on an empty stomach. The researchers reported that taking Absorica LD with a meal didn’t help the drug absorb any faster and had only a “marginal” effect on how much was absorbed.6 This suggests that it may be less important to take Absorica LD with a fatty meal.
Cost of Absorica and Absorica LD: When it comes to cost, both Absorica and Absorica LD are very expensive compared to other forms of isotretinoin on the market. The FDA has recently approved a generic version of Absorica, which is more affordable, but there is no generic version of Absorica LD as yet.
History of Isotretinoin
Gerald Peck and co-workers from the NIH (National Institutes of Health) in Bethesda, Maryland first studied isotretinoin in patients with skin cell disorders. They accidentally found that it also worked on patients with severe acne. Isotretinoin was registered in 1979, released in the United States in 1982 as Accutane, and released in Europe in 1985 as Roaccutane.
Roche’s patent expired in 2002, and manufacturers began selling generic forms of the drug.
In June 2009, shortly after a jury awarded $33 million in damages to people who claimed Accutane caused bowel disease, Roche decided to discontinue selling the brand name, Accutane. The company cited declining sales as their reason.
Topical Isotretinoin
Topical isotretinoin exists but does not produce the results of oral isotretinoin. It is largely of historical significance in acne treatment.
WARNING: Do Not Buy Isotretinoin on the Internet!
According to the FDA:
- “Buying isotretinoin over the Internet bypasses important procedures to ensure that patients can take this drug safely. When these procedures are ignored, isotretinoin can cause serious and harmful side effects.”
- You should NEVER buy isotretinoin without first seeing your healthcare professional.
- You should NEVER take isotretinoin or any of the generic versions of isotretinoin if you are pregnant or trying to get pregnant or could accidentally become pregnant.
- Some websites sell prescription drugs without a prescription. This is illegal and DANGEROUS.
Buying isotretinoin or any other prescription over the Internet often means you will receive pills that contain little or no active ingredient, or in some cases, a different medication entirely. Buying isotretinoin over the Internet is not only illegal, it is potentially dangerous and is also a waste of money.4 I agree strongly with the FDA. NEVER buy isotretinoin over the Internet.”
Dan Kern, Acne.org founder
Presentation of Bias
As a critical sociology major in college, I learned that it is important for an author to present his or her bias. Because we are human and it is impossible to be completely unbiased, the presentation of bias allows the reader to take the author’s bias into account when absorbing content.
My bias: I suffered with moderately severe acne in my adolescence and early adulthood. I took Accutane at age 20 but do not recall the dosage my doctor prescribed. It cleared me up completely within weeks. I transformed from a shy introvert to an outgoing college student. As a result of my skin clearing up, my mental state felt relatively light and good, albeit still somewhat anxious as I had always been. My short-term side effects included severely dry lips, extremely dry skin, dry eyes, and sometimes severe joint pain. I now live with two long-term conditions which may be from taking Accutane or may be coincidence. Since Accutane, whenever I sprint or exert myself in quick bursts my joints react with pain and inflammation, thus limiting my sports endeavors. I also have a mild version of an eye condition called pterygium, which is an irreversible and not-so-attractive growth on the white part of both eyes. My acne relapsed post-Accutane somewhat aggressively to what would be described as moderate acne. I am now able to control my acne symptoms with The Regimen.”
Dan Kern, Acne.org founder
References
How Does Accutane Work?
- Del Rosso, J. Q. Face to face with oral isotretinoin: a closer look at the spectrum of therapeutic outcomes and why some patients need repeated courses. J. Clin. Aesthet. Dermatol. 5, 17 – 24 (2012). https://www.ncbi.nlm.nih.gov/pubmed/23198008
- Ganceviciene, R. & Zouboulis, C. C. Isotretinoin: state of the art treatment for acne vulgaris. J. Dtsch. Dermatol. Ges. 8 Suppl 1, S47 – 59 (2010). https://www.ncbi.nlm.nih.gov/pubmed/20482692
- Dreno, B.et al. An expert view on the treatment of acne with systemic antibiotics and/or oral isotretinoin in the light of the new European recommendations. Eur. J. Dermatol. 16, 565 – 571 (2006). https://www.ncbi.nlm.nih.gov/pubmed/17101480
- Chen, K., White, T. J., Juzba, M. & Chang, E. Oral isotretinoin: an analysis of its utilization in a managed care organization. J. Manag. Care Pharm. 8, 272 – 277 (2002). https://www.ncbi.nlm.nih.gov/pubmed/14613420
- Wysowski, D. K., Swann, J. & Vega, A. Use of isotretinoin (Accutane) in the United States: rapid increase from 1992 through 2000. J. Am. Acad. Dermatol. 46, 505 – 509 (2002). https://www.ncbi.nlm.nih.gov/pubmed/11907498
- Rigopoulos, D., Larios, G. & Katsambas, A. D. The role of isotretinoin in acne therapy: why not as first-line therapy? facts and controversies. Clin. Dermatol. 28, 24 – 30 (2010). https://www.ncbi.nlm.nih.gov/pubmed/20082946
- Dhir, R., Gehi, N. P., Agarwal, R. & More, Y. E. Oral isotretinoin is as effective as a combination of oral isotretinoin and topical anti-acne agents in nodulocystic acne. Indian J. Dermatol. Venereol. Leprol. 74, 187 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18401903
- Nelson, A. M., Gilliland, K. L., Cong, Z. & Thiboutot, D. M. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J. Invest. Dermatol. 126, 2178 – 2189 (2006). https://www.ncbi.nlm.nih.gov/pubmed/16575387
- Plewig, G., Dressel, H., Pfleger, M., Michelsen, S. & Kligman, A. M. Low dose isotretinoin combined with tretinoin is effective to correct abnormalities of acne. J. Dtsch. Dermatol. Ges. 2, 31 – 45 (2004). https://www.ncbi.nlm.nih.gov/pubmed/16281880
- Ellis, C. N. & Krach, K. J. Uses and complications of isotretinoin therapy. J. Am. Acad. Dermatol. 45, S150 – 157 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11606947
- Demircay, Z., Kus, S. & Sur, H. Predictive factors for acne flare during isotretinoin treatment. Eur. J. Dermatol. 18, 452 – 456 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18573721
- Mandekou-Lefaki, I., Delli, F., Teknetzis, A., Euthimiadou, R. & Karakatsanis, G. Low-dose schema of isotretinoin in acne vulgaris. Int. J. Clin. Pharmacol. Res. 23, 41 – 46 (2003). https://www.ncbi.nlm.nih.gov/pubmed/15018017
- Ng, P. P. & Goh, C. L. Treatment outcome of acne vulgaris with oral isotretinoin in 89 patients. Int. J. Dermatol. 38, 213 – 216 (1999). https://www.ncbi.nlm.nih.gov/pubmed/10208621
- Quereux, G., Volteau, C., N’Guyen, J. M. & Dreno, B. Prospective study of risk factors of relapse after treatment of acne with oral isotretinoin. Dermatology 212, 168 – 176 (2006). https://www.ncbi.nlm.nih.gov/pubmed/16484824
- Haryati, I. & Jacinto, S. S. Profile of acne patients in the Philippines requiring a second course of oral isotretinoin. Int. J. Dermatol. 44, 999 – 1001 (2005). https://www.ncbi.nlm.nih.gov/pubmed/16409263
- Akman, A.et al. Treatment of acne with intermittent and conventional isotretinoin: a randomized, controlled multicenter study. Arch. Dermatol. Res. 299, 467 – 473 (2007). https://www.ncbi.nlm.nih.gov/pubmed/17710426
- Morales-Cardona, C. A. & Sanchez-Vanegas, G. Acne relapse rate and predictors of relapse following treatment with oral isotretinoin. Actas Dermosifiliogr. 104, 61 – 66 (2013). https://www.ncbi.nlm.nih.gov/pubmed/22795452
- Rademaker, M., Wishart, J. M. & Birchall, N. M. Isotretinoin 5 mg daily for low-grade adult acne vulgaris–a placebo-controlled, randomized double-blind study. J. Eur. Acad. Dermatol. Venereol. 28, 747 – 754 (2014). https://www.ncbi.nlm.nih.gov/pubmed/23617693
- Boyraz, N. & Mustak, P. K. Comparison of the efficacies of intermittent and continuous low-dose isotretinoin regimens in the treatment of moderate acne vulgaris. Int. J. Dermatol. 52, 1265 – 1267 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23675954
- Borghi, A.et al. Low-cumulative dose isotretinoin treatment in mild-to-moderate acne: efficacy in achieving stable remission. J. Eur. Acad. Dermatol. Venereol. 25, 1094 – 1098 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21198947
- Rasi, A., Behrangi, E., Rohaninasab, M. & Nahad, Z. M. Efficacy of fixed daily 20 mg of isotretinoin in moderate to severe scar prone acne. Adv. Biomed. Res 3, 103 (2014). https://www.ncbi.nlm.nih.gov/pubmed/24804178
- Lee, J. W.et al. Effectiveness of conventional, low-dose and intermittent oral isotretinoin in the treatment of acne: a randomized, controlled comparative study. Br. J. Dermatol. 164, 1369 – 1375 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21114478
- Costa, C. S., Bagatin, E., Martimbianco, A. L. C. et al. Oral isotretinoin for acne. Cochrane Database Syst. Rev. 11, CD009435 (2018). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6383843/
Accutane Side Effects
- Rademaker, M. Adverse effects of isotretinoin: A retrospective review of 1743 patients started on isotretinoin. Australas. J. Dermatol. 51, 248 – 253 (2010). https://www.ncbi.nlm.nih.gov/pubmed/21198520
- Honein, M. A., Paulozzi, L. J. & Erickson, J. D. Continued occurrence of Accutane-exposed pregnancies. Teratology 64, 142 – 147 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11514944
- Brelsford, M. & Beute, T. C. Preventing and managing the side effects of isotretinoin. Semin. Cutan. Med. Surg. 27, 197 – 206 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18786498
- Berard, A.et al. Isotretinoin, pregnancies, abortions and birth defects: a population-based perspective. Br. J. Clin. Pharmacol. 63, 196 – 205 (2007). https://www.ncbi.nlm.nih.gov/pubmed/17214828
- Abroms, L., Maibach, E., Lyon-Daniel, K. & Feldman, S. R. What is the best approach to reducing birth defects associated with isotretinoin? PLoS Med. 3, e483 (2006). https://www.ncbi.nlm.nih.gov/pubmed/17121451
- Shin, J.et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J. Am. Acad. Dermatol. 65, 1117 – 1125 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21565419
- Goulden, V., Layton, A. M. & Cunliffe, W. J. Long-term safety of isotretinoin as a treatment for acne vulgaris. Br. J. Dermatol. 131, 360 – 363 (1994). https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2133.1994.tb08524.x
- McLane, J. Analysis of common side effects of isotretinoin. J. Am. Acad. Dermatol. 45, S188 – 194 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11606952
- Burkhart, C. G. Another threat to the availability of isotretinoin: ocular side effects have aviation authorities considering restricting use from (even potential) pilots. Dermatol. Online J. 14, 2 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18718186
- Barzilai, A., David, M., Trau, H. & Hodak, E. Seborrheic dermatitis-like eruption in patients taking isotretinoin therapy for acne: retrospective study of five patients. Am. J. Clin. Dermatol. 9, 255 – 261 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18572976
- DiGiovanna, J. J. Isotretinoin effects on bone. J. Am. Acad. Dermatol. 45, S176 – 182 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11606950
- DiGiovanna, J. J.et al. Effect of a single course of isotretinoin therapy on bone mineral density in adolescent patients with severe, recalcitrant, nodular acne. J. Am. Acad. Dermatol. 51, 709 – 717 (2004). https://jhu.pure.elsevier.com/en/publications/effect-of-a-single-course-of-isotretinoin-therapy-on-bone-mineral-4
- Eksioglu, E.et al. Sacroiliitis and polyneuropathy during isotretinoin treatment. Clin. Exp. Dermatol. 33, 122 – 124 (2008). https://www.ncbi.nlm.nih.gov/pubmed/17725657
- De Francesco, V., Stinco, G. & Campanella, M. Acute arthritis during isotretinoin treatment for acne conglobata. Dermatology 194, 195 (1997). https://www.ncbi.nlm.nih.gov/pubmed/9094477
- Bewley, A. P., Rankin, E. C., Levell, N. J. & Robinson, T. W. Isotretinoin causing acute aseptic arthropathy. Clin. Exp. Dermatol. 20, 279 (1995). https://www.ncbi.nlm.nih.gov/pubmed/7671431
- Kaplan, G. & Haettich, B. Rheumatological symptoms due to retinoids. Baillieres Clin. Rheumatol. 5, 77 – 97 (1991). https://www.ncbi.nlm.nih.gov/pubmed/2070429
- Hughes, R. A. Arthritis precipitated by isotretinoin treatment for acne vulgaris. J. Rheumatol. 20, 1241 – 1242 (1993). https://www.ncbi.nlm.nih.gov/pubmed/8371229
- Lehucher Ceyrac, D. Acute arthritis after isotretinoin. Dermatology 198, 406 – 407 (1999). https://www.ncbi.nlm.nih.gov/pubmed/10449946
- Kaymak, Y. Creatine phosphokinase values during isotretinoin treatment for acne. Int. J. Dermatol. 47, 398 – 401 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18377609
- Kaymak, Y., Taner, E. & Taner, Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int. J. Dermatol. 48, 41 – 46 (2009). https://www.ncbi.nlm.nih.gov/pubmed/19126049
- Sundstrom, A.et al. Association of suicide attempts with acne and treatment with isotretinoin: retrospective Swedish cohort study. BMJ 341, c5812 (2010). https://www.bmj.com/content/341/bmj.c5812
- Laroche, M. L., Macian-Montoro, F., Merle, L. & Vallat, J. M. Cerebral ischemia probably related to isotretinoin. Ann. Pharmacother. 41, 1073 – 1076 (2007). https://www.ncbi.nlm.nih.gov/pubmed/17472998
- Zane, L. T., Leyden, W. A., Marqueling, A. L. & Manos, M. M. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch. Dermatol. 142, 1016 – 1022 (2006). https://www.ncbi.nlm.nih.gov/pubmed/16924051
- Karadag, A. S., Ertugrul, D. T., Tutal, E. & Akin, K. O. Short-term isotretinoin treatment decreases insulin- like growth factor-1 and insulin-like growth factor binding protein-3 levels: does isotretinoin affect growth hormone physiology? Br. J. Dermatol. 162, 798 – 802 (2010). https://www.ncbi.nlm.nih.gov/pubmed/20128787
- Ozdemir, M. A.et al. Isotretinoin-induced agranulocytosis. Pediatr. Dermatol. 24, 425 – 426 (2007). https://www.ncbi.nlm.nih.gov/pubmed/17845176
- Reddy, D., Siegel, C. A., Sands, B. E. & Kane, S. Possible association between isotretinoin and inflammatory bowel disease. Am. J. Gastroenterol. 101, 1569 – 1573 (2006). https://www.ncbi.nlm.nih.gov/pubmed/16863562
- Crockett, S. D., Porter, C. Q., Martin, C. F., Sandler, R. S. & Kappelman, M. D. Isotretinoin use and the risk of inflammatory bowel disease: a case-control study. Am. J. Gastroenterol. 105, 1986 – 1993 (2010). https://www.ncbi.nlm.nih.gov/pubmed/20354506
- Erturan, I., Naziroglu, M. & Akkaya, V. B. Isotretinoin treatment induces oxidative toxicity in blood of patients with acne vulgaris: a clinical pilot study. Cell Biochem. Funct. 30, 552 – 557 (2012). https://www.ncbi.nlm.nih.gov/pubmed/22517509
- Neudorfer, M.et al. Ocular adverse effects of systemic treatment with isotretinoin. Arch. Dermatol. 148, 803 – 808 (2012). https://jamanetwork.com/journals/jamadermatology/fullarticle/1148708
- Alhusayen, R. O.et al. Isotretinoin use and the risk of inflammatory bowel disease: a population-based cohort study. J. Invest. Dermatol. 133, 907 – 912 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23096714
- Etminan, M., Bird, S. T., Delaney, J. A., Bressler, B. & Brophy, J. M. Isotretinoin and risk for inflammatory bowel disease: a nested case-control study and meta-analysis of published and unpublished data. JAMA Dermatol. 149, 216 – 220 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23426479
- Stobaugh, D. J., Deepak, P. & Ehrenpreis, E. D. Alleged isotretinoin-associated inflammatory bowel disease: disproportionate reporting by attorneys to the Food and Drug Administration Adverse Event Reporting System. J. Am. Acad. Dermatol. 69, 393 – 398 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23683730
- Gungor, S. & Gokdemir, G. Anal fissure and rectal bleeding as a complication of systemic isotretinoin therapy: dermatologists know this side-effect, what about proctologists? Colorectal Dis. 15, 1187 – 1188 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23701373
- Javanbakht, A. M., Pour, H. M. & Tarrahic, M. J. Effects of oral isotretinoin on serum folic acid levels. J. Drugs Dermatol. 11, e23 – 24 (2012). https://www.ncbi.nlm.nih.gov/pubmed/23135667
- Akturk, A. S.et al. Effects of isotretinoin on serum vitamin E levels in patients with acne. Int. J. Dermatol. 52, 363 – 366 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23414163
- Bettoli, V., Guerra-Tapia, A., Herane, M. I. & Piquero-Martín, J. Challenges and solutions in oral isotretinoin in acne: Reflections on 35 years of experience. Clin. Cosmet. Investig. Dermatol. 12, 943-951 (2019). https://pubmed.ncbi.nlm.nih.gov/32021364/
- Acmaz, G., Cınar, L., Acmaz, B. et al. The effects of oral isotretinoin in women with acne and polycystic ovary syndrome. Biomed. Res. Int. 2019, 2513067 (2019). https://pubmed.ncbi.nlm.nih.gov/31080813/
- Evaristo, L. S. B. F. & Bagatin, E. Use of oral isotretinoin to treat acne in the public system: a hospital-based retrospective cohort. Sao Paulo Med J. 137, 363-368 (2019). https://pubmed.ncbi.nlm.nih.gov/31691769/
Pregnancy and Accutane
- CDC. Epidemiologic Notes and Reports Isotretinoin — A Newly Recognized Human Teratogen. Morbidity and Mortality Weekly Report 33, 171 – 173 (1986). https://www.cdc.gov/mmwr/preview/mmwrhtml/00000310.htm
- Hull, P. R. & D’Arcy, C. Acne, depression, and suicide. Dermatol Clin 23, 665 – 674 (2005). https://www.ncbi.nlm.nih.gov/pubmed/16112443
- Magin, P., Pond, D. & Smith, W. Isotretinoin, depression and suicide: a review of the evidence. Br. J. Gen. Pract. 55, 134 – 138 (2005). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1463189/
- Kaymak, Y., Taner, E. & Taner, Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int. J. Dermatol. 48, 41 – 46 (2009). https://www.ncbi.nlm.nih.gov/pubmed/19126049
- Lowery, K., Rosen, T. & Malek, J. iPLEDGE must abstain from abstinence. J. Clin. Aesthet. Dermatol. 13, 54-56 (2020). https://pubmed.ncbi.nlm.nih.gov/32884622/
- Henry, D. et al. Occurrence of pregnancy and pregnancy outcomes during isotretinoin therapy. CMAJ 188, 723 – 730 (2016). https://www.ncbi.nlm.nih.gov/pubmed/27114489
- Zomerdijk, I. M. et al. Isotretinoin exposure during pregnancy: a population-based study in The Netherlands. BMJ Open 4, e005602 (2014). https://www.ncbi.nlm.nih.gov/pubmed/25392022
- Berard, A. et al. Isotretinoin, pregnancies, abortions and birth defects: a population-based perspective. Br. J. Clin. Pharmacol. 63, 196 – 205 (2007). https://www.ncbi.nlm.nih.gov/pubmed/17214828
- Collins, M. K. et al. Compliance with pregnancy prevention measures during isotretinoin therapy. J. Am. Acad. Dermatol. 70, 55 – 59 (2014). https://www.ncbi.nlm.nih.gov/pubmed/24157382
- Tkachenko, E., Singer, S., Sharma, P., Barbieri, J. & Mostaghimi, A. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 155, 1175-1179 (2019). https://pubmed.ncbi.nlm.nih.gov/31314041/
- Charrow, A., Xia, F. D., Lu, J., Waul, M., Joyce, C. & Mostaghimi, A. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PLoS One. 14, e0210445 (2019). https://pubmed.ncbi.nlm.nih.gov/30913210/
- Shin, J. et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J. Am. Acad. Dermatol. 65, 1117 – 1125 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21565419
Suicide and Depression
- Chandrasekaran, S., De Sousa, J. F. M., Paghdar, S., Khan, T. M., Patel, N. P. & Tsouklidis, N. Is isotretinoin in acne patients a psychological boon or a bane: A systematic review. Cureus. 13, e16834 (2021). https://pubmed.ncbi.nlm.nih.gov/34513424/
- Jacobs, D. G., Deutsch, N. L. & Brewer, M. Suicide, depression, and isotretinoin: is there a causal link? J. Am. Acad. Dermatol. 45, S168 – 175 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11606949
- Jick, S. S., Kremers, H. M. & Vasilakis-Scaramozza, C. Isotretinoin use and risk of depression, psychotic symptoms, suicide, and attempted suicide. Arch. Dermatol. 136, 1231 – 1236 (2000). https://www.ncbi.nlm.nih.gov/pubmed/11030769
- Hull, P. R. & D’Arcy, C. Acne, depression, and suicide. Dermatol. Clin. 23, 665 – 674 (2005). https://www.ncbi.nlm.nih.gov/pubmed/16112443
- Magin, P., Pond, D. & Smith, W. Isotretinoin, depression and suicide: a review of the evidence. Br. J. Gen. Pract. 55, 134 – 138 (2005). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1463189/
- Kaymak, Y., Taner, E. & Taner, Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int. J. Dermatol. 48, 41 – 46 (2009). https://www.ncbi.nlm.nih.gov/pubmed/19126049
- Sundstrom, A.et al. Association of suicide attempts with acne and treatment with isotretinoin: retrospective Swedish cohort study. BMJ 341, c5812 (2010). https://www.bmj.com/content/341/bmj.c5812
- Chia, C. Y., Lane, W., Chibnall, J., Allen, A. & Siegfried, E. Isotretinoin therapy and mood changes in adolescents with moderate to severe acne: a cohort study. Arch. Dermatol. 141, 557 – 560 (2005). https://www.ncbi.nlm.nih.gov/pubmed/15897376
- Hersom, K., Neary, M. P., Levaux, H. P., Klaskala, W. & Strauss, J. S. Isotretinoin and antidepressant pharmacotherapy: a prescription sequence symmetry analysis. J. Am. Acad. Dermatol. 49, 424 – 432 (2003). https://www.ncbi.nlm.nih.gov/pubmed/12963905
- Ferahbas, A.et al. A pilot study evaluating anxiety and depressive scores in acne patients treated with isotretinoin. J. Dermatolog. Treat. 15, 153 – 157 (2004). https://www.ncbi.nlm.nih.gov/pubmed/15204147
- O’Reilly, K. C., Shumake, J., Gonzalez-Lima, F., Lane, M. A. & Bailey, S. J. Chronic administration of 13-cis- retinoic acid increases depression-related behavior in mice. Neuropsychopharmacology 31, 1919 – 1927 (2006). https://www.ncbi.nlm.nih.gov/pubmed/16395305
- Azoulay, L., Blais, L., Koren, G., LeLorier, J. & Berard, A. Isotretinoin and the risk of depression in patients with acne vulgaris: a case-crossover study. J. Clin. Psychiatry 69, 526 – 532 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18363422
- Yesilova, Y., Bez, Y., Ari, M., Kaya, M. C. & Alpak, G. Effects of isotretinoin on obsessive compulsive symptoms, depression, and anxiety in patients with acne vulgaris. J. Dermatolog. Treat 23, 268 – 271 (2012). https://www.ncbi.nlm.nih.gov/pubmed/21815780
- Ergun, T. et al. Isotretinoin has no negative effect on attention, executive function and mood. J. Eur. Acad. Dermatol. Venereol. 26, 431 – 439 (2012). https://www.ncbi.nlm.nih.gov/pubmed/21545542
- Marron, S. E., Tomas-Aragones, L. & Boira, S. Anxiety, depression, quality of life and patient satisfaction in acne patients treated with oral isotretinoin. Acta Derm. Venereol. 93, 701 – 706 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23727704
- Nevoralova, Z. & Dvorakova, D. Mood changes, depression and suicide risk during isotretinoin treatment: a prospective study. Int. J. Dermatol. 52, 163 – 168 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23347302
- Yesilova, Y., Bez, Y., Ari, M. & Turan, E. Effects of isotretinoin on social anxiety and quality of life in patients with acne vulgaris: a prospective trial. Acta Dermatovenerol. Croat. 20, 80 – 83 (2012). https://www.ncbi.nlm.nih.gov/pubmed/22726279
- Cohen, J., Adams, S. & Patten, S. No association found between patients receiving isotretinoin for acne and the development of depression in a Canadian prospective cohort. Can. J. Clin. Pharmacol. 14, e227 – 233 (2007). https://www.ncbi.nlm.nih.gov/pubmed/17556790
- Wysowski, D. K., Pitts, M. & Beitz, J. Depression and suicide in patients treated with isotretinoin. N. Engl. J. Med. 344, 460 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11221610
- Marqueling, A. L. & Zane, L. T. Depression and suicidal behavior in acne patients treated with isotretinoin: a systematic review. Semin. Cutan. Med. Surg. 26, 210 – 220 (2007). https://www.ncbi.nlm.nih.gov/pubmed/18395669
- Wysowski, D. K. & Beitz, J. Methodological limitations of the study Isotretinoin use and risk of depression, psychotic symptoms, suicide, and attempted suicide. Arch. Dermatol. 137, 1102 – 1103 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11493109
- Strahan, J. E. & Raimer, S. Isotretinoin and the controversy of psychiatric adverse effects. Int. J. Dermatol. 45, 789 – 799 (2006). https://www.ncbi.nlm.nih.gov/pubmed/16863513
- Wysowski, D. K., Pitts, M. & Beitz, J. An analysis of reports of depression and suicide in patients treated with isotretinoin. J. Am. Acad. Dermatol. 45, 515 – 519 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11568740
- Li, C., Chen, J., Wang, W., Ai, M., Zhang, Q. & Kuang, L. Use of isotretinoin and risk of depression in patients with acne: a systematic review and meta-analysis [published correction appears in BMJ Open. 9, e021549corr1 (2019)]. BMJ Open. 9, e021549 (2019). https://pubmed.ncbi.nlm.nih.gov/30670500/
- Wolverton, S. E. & Harper, J. C. Important controversies associated with isotretinoin therapy for acne. Am. J. Clin. Dermatol. 14, 71 – 76 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23559397
- Misery, L.et al. [Isotretinoin and adolescent depression]. Ann. Dermatol. Venereol. 139, 118 – 123 (2012). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4804262/
- Thiboutot, D. & Zaenglein, A. Isotretinoin and affective disorders: thirty years later. J. Am. Acad. Dermatol. 68, 675 – 676 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23522408
Take with a High Fat Meal
- Roche Laboratories, I. Accutane (isotretinoin) capsule, liquid filled https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=8655#section-13
- Colburn, W. A., Gibson, D. M., Wiens, R. E. & Hanigan, J. J. Food increases the bioavailability of isotretinoin. J. Clin. Pharmacol. 23, 534 – 539 (1983). https://www.ncbi.nlm.nih.gov/pubmed/6582073
- Webster, G. F., Leyden, J. J. & Gross, J. A. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, 4-treatment, crossover study. J. Am. Acad. Dermatol. 69, 762 – 767 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23953888
- ABSORICA [prescribing information]. (Ranbaxy Laboratories I., Jacksonville, FL, 2012). https://www.absorica.com/hcp/references/
- Bellomo, R., Brunner, M. & Tadjally, E. New formulations of isotretinoin for acne treatment: Expanded options and clinical implications. J. Clin. Aesthet. Dermatol. 14, S18-S23 (2021). https://pubmed.ncbi.nlm.nih.gov/35291260/
- Madan, S., Kumar, S. & Segal, J. Comparative pharmacokinetic profiles of a novel low-dose micronized-isotretinoin 32 mg formulation and lidose-isotretinoin 40 mg in fed and fasted conditions: Two open-label, randomized, crossover studies in healthy adult participants. Acta Derm Venereol. 100, adv00049 (2020). https://pubmed.ncbi.nlm.nih.gov/31774544/
 Acne.org Products
Acne.org Products