Yes. Contrary to Popular Belief, Today’s Micronized Zinc Oxide and Titanium Dioxide Sunscreens Primarily Absorb UV Radiation, Just like Their Chemical Sunscreen Cousins

The Essential Info
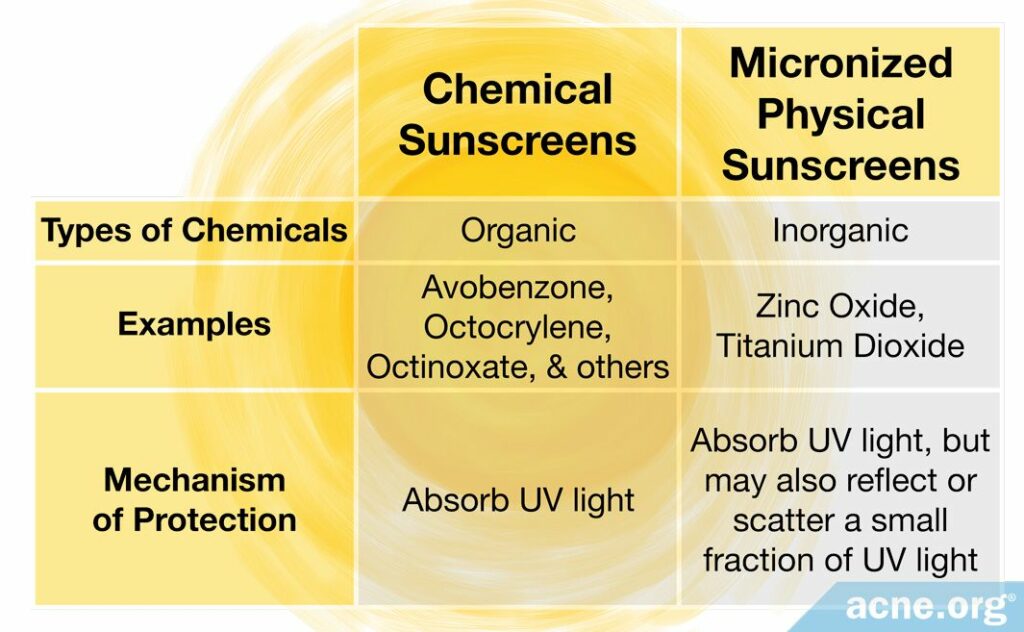
In the past, chemical sunscreens (avobenzone, homosalate, octinoxate, octisalate, octocrylene, oxybenzone) worked by absorbing the sun’s rays, while physical sunscreens (zinc oxide and titanium dioxide) commonly reflected and scattered the sun’s rays.
Today, with the advent of micronized physical sunscreens, both chemical and physical sunscreens now protect the skin from the sun mainly by absorbing UV light.
Excessive sun exposure can be damaging to the skin, which is why it is a good idea to wear sunscreen when spending long periods of time outside. This is particularly important if you are undergoing certain acne treatments, such as isotretinoin (Accutane®) and antibiotics, which can increase the skin’s sensitivity to the sun.

The Science
Chemical and Physical Sunscreen Active Ingredients
- Chemical sunscreen ingredients: So-called “chemical” sunscreen active ingredients include all sunscreen ingredients except for zinc oxide and titanium dioxide. The ones most commonly used in the United States are (in alphabetical order):
- Avobenzone
- Homosalate
- Octinoxate
- Octisalate
- Octocrylene
- Oxybenzone
- Physical sunscreen ingredients: So-called “physical” sunscreen ingredients include only these two:
- Titanium dioxide
- Zinc oxide
Many people believe that chemical sunscreen ingredients protect the skin by absorbing UV light, whereas physical sunscreen ingredients do not absorb UV light but, instead, reflect and scatter it. This was true in the past, but is no longer the case. Let’s look at it in historical context so it makes more sense.
Chemical sunscreen ingredients: Historically, chemical sunscreens have always worked by absorbing UV light before it can reach the skin, and continue to work in this same way.
Physical sunscreen ingredients: Historically, manufacturers were only able to produce zinc oxide and titanium dioxide in large particle sizes that appeared opaque/white when applied on the skin. Because they had such large particle sizes, they were able to reflect and scatter UV light away from the skin, and also absorb some UV light. However, since the advent of smaller, micronized zinc oxide and titanium dioxide particles that allow the sunscreen to appear more-or-less transparent when applied on the skin, they now work primarily by absorbing UV light.

So, in today’s sunscreens, both chemical and physical sunscreen ingredients work primarily by absorbing UV light.
In the real world, the right mix of sunscreen ingredients of either type will protect you from the sun, but it is important to reapply sunscreen every two hours or so to maintain adequate UV protection for your skin. This is particularly vital with chemical sunscreen ingredients, which break down over time in the sun.
How chemical and physical sunscreen ingredients protect the skin from the sun
Even though both chemical and physical sunscreen ingredients protect the skin mainly by absorbing UV rays, there remain subtle differences in how they work. Let’s look at this more closely.
Whenever sunlight hits any substance, there are three possibilities for what can happen:
- The substance can absorb the light
- The substance can reflect the light
- The substance can scatter the light1
- Chemical sunscreen ingredients: When it comes to sunscreens, chemical sunscreen ingredients only absorb UV light.
- Physical sunscreen ingredients: On the other hand, today’s physical sunscreen ingredients primarily absorb UV light, but physical sunscreen ingredients also reflect and scatter some of the UV light.
Both chemical and physical sunscreens consist of chemicals
Actually, the terms “chemical” and “physical” are somewhat misleading, because both types of sunscreens consist of chemicals. In fact, every substance is made of chemicals, including water. However, the chemicals that chemical and physical sunscreens contain are different.
- Chemical sunscreen ingredients: So-called “chemical” sunscreen ingredients consist of organic chemicals, which means substances that contain carbon.
- Physical sunscreen ingredients: So-called “physical” sunscreen ingredients consist of inorganic chemicals–in other words, substances that do not contain carbon.
Thus, a more exact name for chemical sunscreen ingredients would be “organic” sunscreen ingredients, and a more exact name for physical sunscreen ingredients would be “inorganic” sunscreen ingredients.
This is an important difference, because organic and inorganic chemicals tend to behave in unique ways, and this also applies to sunscreen ingredients. In other words, although chemical and physical sunscreen ingredients both work primarily by absorbing UV light, they accomplish this in different ways.
- Chemical sunscreen ingredients:
- Chemical sunscreen ingredients are molecules that contain a special structure called a chromophore. This structure absorbs UV light.
- Some sunscreen ingredients contain a chromophore that only absorbs UVA light. Others contain a chromophore that only absorbs UVB light. For this reason, manufacturers often combine multiple chemical sunscreen ingredients to create a broad-spectrum sunscreen, which protects the skin from both UVA and UVB rays.
- Some chemical sunscreen ingredients are photostable, which means they continue to function well after long periods of exposure to UV light.
- Other chemical sunscreen ingredients are photounstable or photolabile, which means they break down after some time in the sun. Once these ingredients break down, they no longer protect the skin from UV light. This is the main reason why it is important to reapply sunscreen that contains chemical sunscreen ingredients every two hours or so.
- Physical sunscreen ingredients:
- Physical sunscreen ingredients are actually tiny crystals. These crystals absorb UV light.
- The size of the crystals determines how much light, if any, the physical sunscreen ingredients reflect and scatter. Small crystals mostly absorb UV light. Larger crystals, such as those used in physical sunscreens in the past, reflect and scatter more light.
- All physical sunscreen ingredients are photostable, which means that, unlike some chemical sunscreen ingredients, they do not break down after exposure to UV light
- Some physical sunscreen ingredients are photoreactive, which means they can react with chemicals in the air or with other ingredients in the sunscreen formulation to form harmful substances called free radicals. However, manufacturers today usually coat physical sunscreen crystals with non-reactive substances to prevent this.
How chemical sunscreen ingredients protect the skin from the sun: The full scoop
To understand how chemical sunscreen ingredients absorb UV light, we need to zoom in on them. At the microscopic level, chemical sunscreen ingredients consist of large organic molecules. The part of the sunscreen molecule that absorbs UV light is called the chromophore.2
The chromophore consists of a ring of atoms connected to each other by chemical bonds. To visualize the chromophore, let’s take a quick detour into chemistry.
Atoms contain tiny negatively charged particles called electrons. Two atoms can form a chemical bond, which “glues” them to each other, by sharing a pair of electrons. This type of bond is called a “single” bond. You can imagine this as two people standing side by side and holding hands.
Sometimes, two atoms share two pairs of electrons, forming a so-called “double” bond. A double bond is stronger than a single bond. You can picture this as two people standing side by side, and not only holding hands, but also tying their shoelaces together so that one person’s right shoe is linked to the other person’s left shoe.
The chromophore of a chemical sunscreen ingredient is a ring of atoms that are connected by alternating double and single bonds.2 For example, avobenzone, a common chemical sunscreen ingredient, contains six atoms arranged in a ring like this. Using our analogy, imagine six people standing in a circle and holding hands. Each person in the circle is connected to one of his neighbors by a single bond–in other words, only by holding hands, and to the other neighbor by a double bond–in other words, by holding hands and tying their shoelaces together.
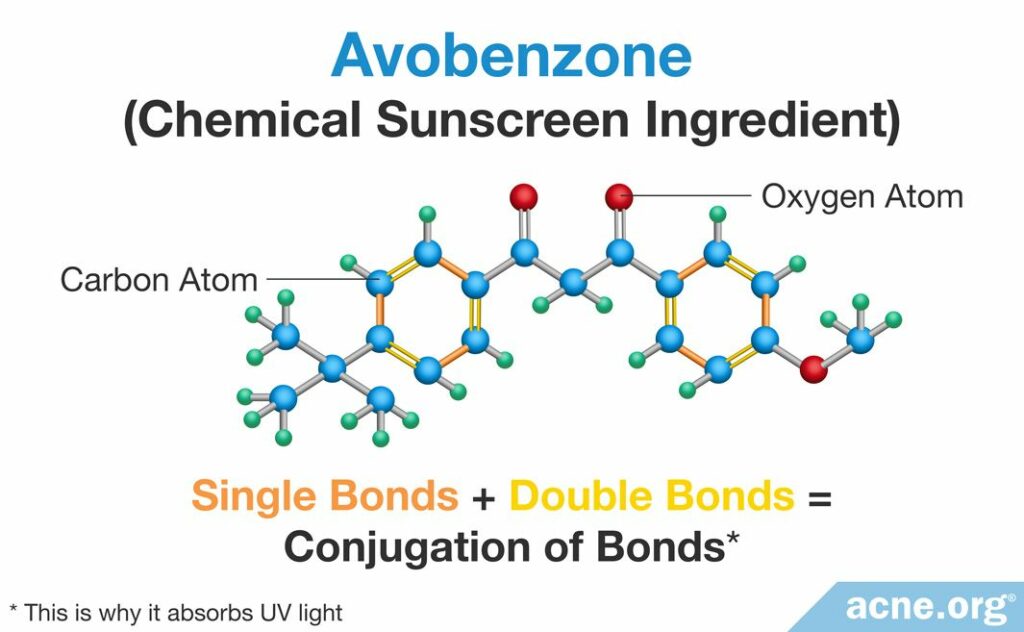
This alternation of single and double bonds is called conjugation of bonds, and this is what allows chemical sunscreen ingredients to absorb UV light.
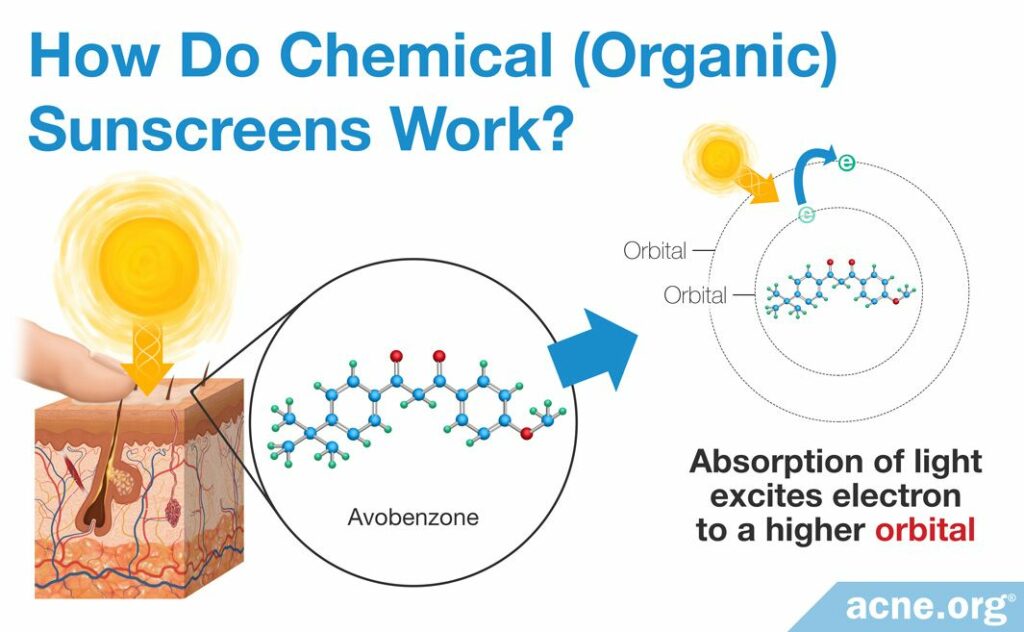
So how exactly do conjugated bonds allow a chemical sunscreen to absorb UV light? Remember that a bond consists of electrons that are shared between two neighboring atoms. Each of those electrons is not sitting still in one place, but instead is circling around the nucleus, or center, of its atom along a pathway called an orbital. This is analogous to the way a satellite circles around the Earth along its orbit.
As you probably know, UV light, and indeed any light, is a form of energy. Therefore, when a sunscreen molecule absorbs UV light, it absorbs energy. The electrons in the conjugated bonds use this extra energy to “jump” to a higher orbital, farther away from the center of the atom. Think of a rocket that is launching a satellite into orbit: the father away from the Earth we want the satellite to go, the more energy the rocket is going to need. In the sunscreen molecule, the electrons are the satellites, and the UV light is the rocket that launches them farther away from the center of their atoms.2
When a sunscreen molecule absorbs UV light and the electrons in the sunscreen molecule jump to higher orbitals, we say that the sunscreen molecule becomes “excited.” Of course, as the saying goes, “what goes up must come down.” In other words, eventually the electrons will have to return to their original orbitals, and the sunscreen molecule will have to get rid of the extra energy. When this occurs, we say that the sunscreen molecule has returned to its “ground state.”2
The number of conjugated bonds determines what kind of UV light the sunscreen molecule can absorb. Ultraviolet light comes in a whole range of wavelengths: UVA light has longer wavelengths, while UVB light has shorter wavelengths. Sunscreen molecules with a small number of conjugated bonds do a better job of absorbing shorter-wavelength UV light–in other words, UVB light. For example, octinoxate absorbs UVB light better than UVA light.2,3
On the other hand, sunscreen molecules with a larger number of conjugated bonds do a better job of absorbing longer-wavelength UV light. For example, avobenzone absorbs UVA light better than UVB light.2,3
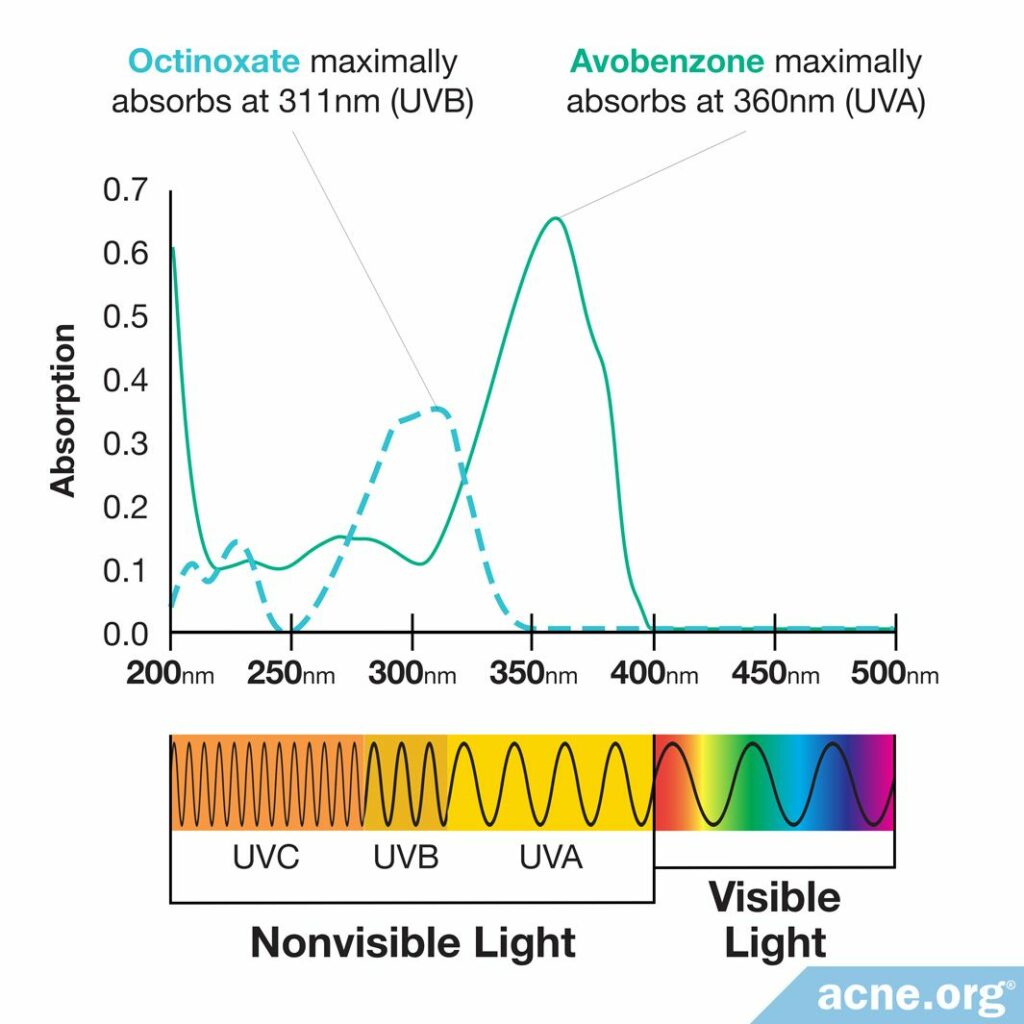
To make sure that chemical sunscreens provide protection from both UVA and UVB light, manufacturers often combine different ingredients to make a so-called broad-spectrum sunscreen.
As we have seen, when a chemical sunscreen molecule absorbs UV light, it absorbs energy, which the sunscreen molecule must get rid of in order to return to the ground state. Physics tells us that energy cannot be created or destroyed, but it can be converted from one form to another. Chemical sunscreen ingredients deal with the energy they absorb from UV light in one of two ways:
1. Photostable sunscreen ingredients: Some chemical sunscreen ingredients convert light energy into heat and release it. In this way, the sunscreen gets rid of the absorbed energy and goes back to its original state. This process can happen over and over again as the sunscreen ingredient absorbs more UV energy, converts it, and releases it as heat. This type of sunscreen ingredient is called photostable.
2. Photounstable sunscreen ingredients: Some chemical sunscreen ingredients change their structure when they absorb UV light. In other words, the light energy causes the chemicals in the sunscreen to break down so that, over time, the sunscreen loses its ability to protect us from the sun. This type of chemical sunscreen ingredient is called photounstable or photolabile.2,4-6
Avobenzone is an example of a photolabile chemical sunscreen ingredient. To extend the lifetime of photolabile sunscreen ingredients, manufacturers often add chemicals called photostabilizers, which prevent the sunscreen ingredients from breaking down. Still, all chemical sunscreen ingredients tend to degrade over time in the sun, even if they are stabilized. This is one of the main reasons you need to reapply a chemical sunscreen every two hours or so to maintain adequate sun protection.
How physical sunscreens protect the skin from the sun: The full scoop
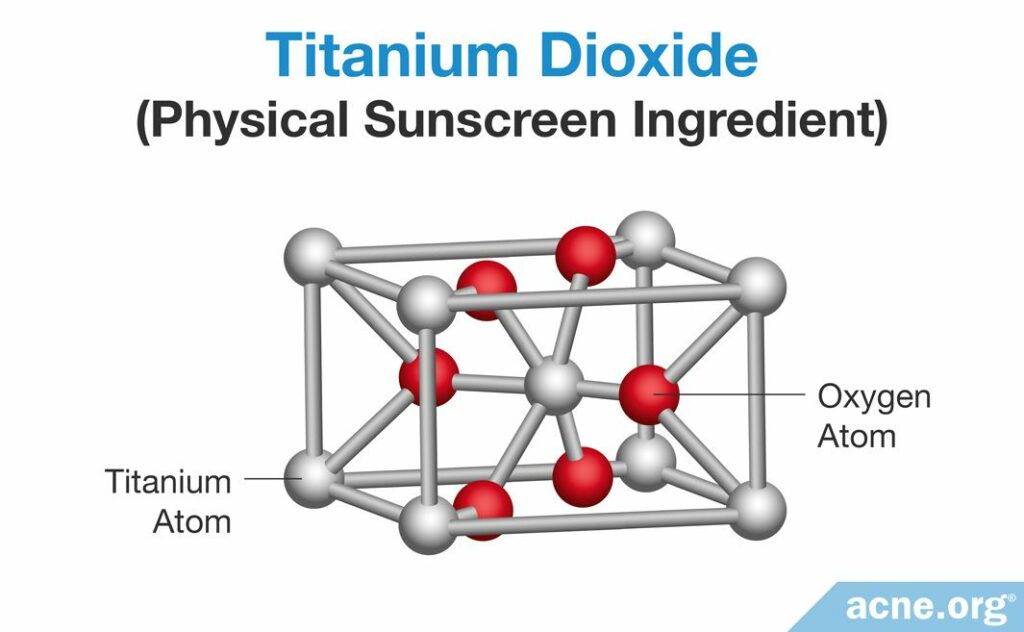
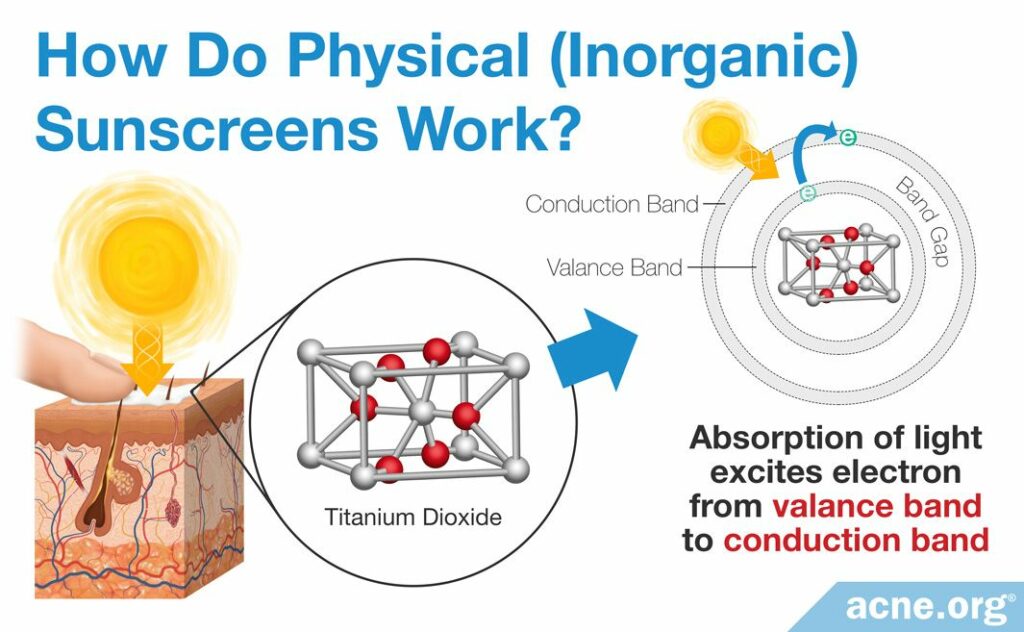
Just as with chemical sunscreens, to understand how physical sunscreens absorb UV light, we need to zoom in on their ingredients. As we know, physical sunscreens consist of ingredients made from metal and oxygen. At the microscopic level, these atoms form three-dimensional structures called crystal structures.
For example, titanium dioxide can form three different types of crystals, called rutile, anatase, and brookite.6 People obtain these crystals by processing natural metal ores. The picture below shows an example of rutile.
In the case of zinc oxide, the two common crystal forms are mineral wurtzite and zinc-blende.7 Wurtzite is the form usually used in physical sunscreens.
Similar to chemical sunscreen ingredients, physical sunscreen ingredients contain electrons that jump to a higher energy level when they absorb UV light.
What defines the absorptive properties of physical sunscreens, though, is particle size rather than alternating single and double bonds, as seen in chemical sunscreens. Just like in chemical sunscreen ingredients, in a physical sunscreen ingredient each electron is normally circling around the center of its atom along an orbital. However, when an electron in a physical sunscreen ingredient absorbs light energy, it can jump out of its orbital into what is called a conduction band.1,5,6,8,9 This is a band around the whole crystal where electrons from multiple atoms are moving around. You can visualize this as a swarm of insects flying around the edges of an ice cube. Of course, just like in chemical sunscreen ingredients, the electrons eventually return to their lower energy state, called the ground state, and the physical sunscreen ingredient gets rid of the extra energy as heat.1,10
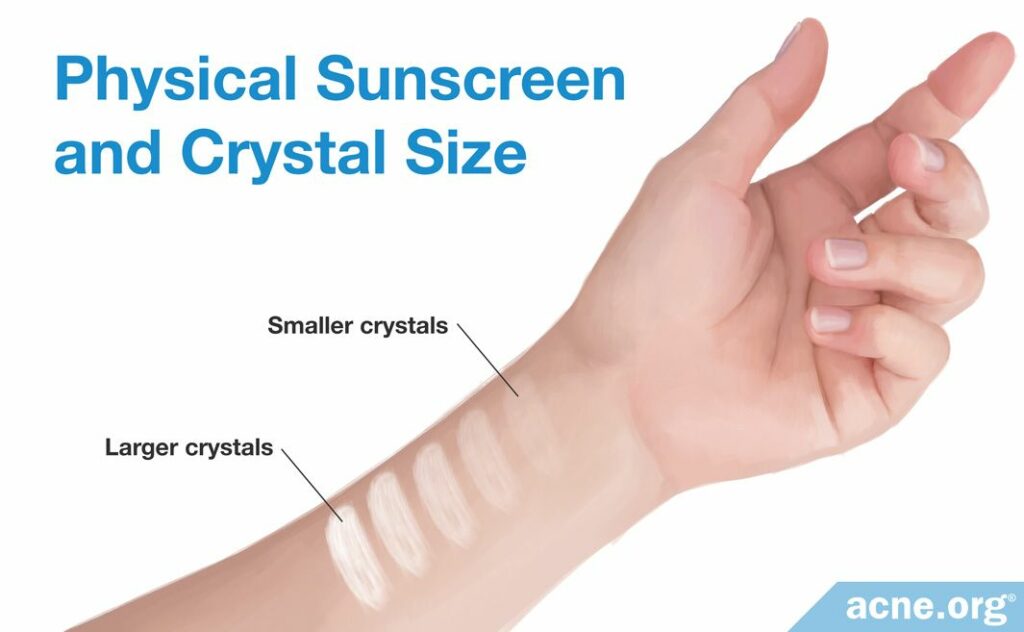
How much UV light a physical sunscreen ingredient absorbs depends on how large the crystals are.
- Smaller crystals tend to absorb more UV light
- Larger particles reflect and scatter more light5,6,8
In the past, when physical sunscreens contained titanium dioxide crystals over 200 nanometers in size–which is still very tiny, but large for a crystal–they mostly reflected and scattered light, including visible light. This is why putting on these sunscreens made people’s skin look ghostly white. Today, manufacturers avoid this effect by making extremely small crystals, which measure only 10 – 30 nanometers. These crystals mostly absorb UV light and do not reflect visible light, so they look more transparent on the skin.5,6,8

A study published in the journal Photodermatology, Photoimmunology, and Photomedicine in 2016 showed that modern physical sunscreen ingredients, with tiny crystals, absorb the vast majority of UV light, just like chemical sunscreen ingredients do. These physical sunscreen ingredients only reflect and scatter about five percent of UVB light, while absorbing the rest. The researchers wrote, “The average range of reflection for zinc oxide and titanium dioxide throughout the UV range was only 4 – 5%…The remainder of the UV protection is provided by…absorbance of the UV photos.”9
Like many chemical sunscreen ingredients, when physical sunscreen ingredients absorb UV light energy, they later release it as heat.1,10 However, unlike chemical sunscreen ingredients, physical sunscreen ingredients do not degrade in the presence of UV light. In other words, they are photostable.4,6 Zinc oxide is particularly stable. Even though physical sunscreen ingredients do not break down in the sun, it is still a good idea to reapply a physical sunscreen every two hours or so. This is because most people do not apply enough sunscreen in the first place, and also because sunscreens tend to wash off during swimming and physical activities that induce sweating.
Although physical sunscreen ingredients are photostable, they can still be photoreactive. This means that when physical sunscreen ingredients absorb UV energy, they may react with other substances in their surroundings, such as other ingredients in the sunscreen product, oxygen in the air, or proteins or oils from our skin. These reactions can produce harmful substances called free radicals, which can damage the cells in our skin.1,2,5,10 However, most manufacturers now coat physical sunscreen crystals with non-reactive materials like aluminum oxide so as to prevent the formation of free radicals.1,5

According to an article published in 2007 in the Indian Journal of Dermatology, Venereology and Leprology, “Although [physical sunscreens] are not inert per se, they can be coated to make them stable, non-toxic and safe.”1
Lastly, scientists have wondered whether tiny crystals of physical sunscreen ingredients might be dangerous because they could be absorbed into the skin. However, current evidence shows that even nano-sized titanium dioxide and zinc oxide crystals remain on top of the skin, where there are only dead skin cells. The crystals do not reach living cells in deeper layers of the skin, and are therefore not harmful.1,10
References
- More, B. D. Physical sunscreens: on the comeback trail. Indian J Dermatol Venereol Leprol 73, 80 – 5 (2007). http://www.ijdvl.com/article.asp?issn=0378-6323;year=2007;volume=73;issue=2;spage=80;epage=85;aulast=More
- Forestier, S. Rationale for sunscreen development. J Am Acad Dermatol 58, S133 – 138 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18410799
- Dondi, D., Albini, A. & Serpone, N. Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection. Photochem Photobiol Sci 5, 835 – 843 (2006). https://www.ncbi.nlm.nih.gov/pubmed/17047836
- Mancebo, S. E., Hu, J. Y. & Wang, S. Q. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin 32, 427 – 38 (2014). https://www.ncbi.nlm.nih.gov/pubmed/24891063
- Lim, H. W., Honigsmann, H. & Hawk, J. L. Photodermatology (Informa Healthcare, New York, 2007). https://books.google.com/books?id=AFXOAwAAQBAJ&pg=PA274&lpg=PA274&dq=Lim,+H.+W.,+Honigsmann,+H.+%26+Hawk,+J.+L. Photodermatology (Informa+Healthcare,+New+York,+2007).&source=bl&ots=dfCN7tERhd&sig=ACfU3U1E2ltapW_UNkPXiBipPIhtL3qe7w&hl=en&sa=X&ved=2ahUKEwj5itetod3oAhVUBc0KHYlMAG8Q6AEwAHoECAsQKQ#v=onepage&q=Lim%2C H. W.%2C Honigsmann%2C H. %26 Hawk%2C J. L. Photodermatology (Informa Healthcare%2C New York%2C 2007).&f=false
- Manaia, E. B., Kaminski, R. C., Corrêa, M. A. & Chiavacci, L. A. Inorganic UV filters. Brazilian J Pharm Sci 49, 201 – 209 (2013). http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1984-82502013000200002
- Smijs, T. G. & Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl 13, 95 – 112 (2011). https://www.ncbi.nlm.nih.gov/pubmed/24198489
- Lim, H. W. & Draelos, Z. K. Clinical guide to sunscreens and photoprotection / edited by Henry W. Lim, Zoe Diana Draelos. (Informa Healthcare, New York, 2009). https://www.academia.edu/32008242/Clinical_Guide_to_Sunscreens_and_Photoprotection
- Cole, C., Shyr, T. & Ou-Yang, H. Metal oxide sunscreens protect skin by absorption, not by reflection or scattering. Photodermatol Photoimmunol Photomed 32, 5 – 10 (2016). https://www.ncbi.nlm.nih.gov/pubmed/26431814
- Mitchnick, M. A., Fairhurst, D. & Pinnell, S. R. Microfine zinc oxide (Z-cote) as a photostable UVA/UVB sunblock agent. J Am Acad Dermatol 40, 85 – 90 (1999). https://www.ncbi.nlm.nih.gov/pubmed/9922017
 Acne.org Products
Acne.org Products