Yes, Benzoyl Peroxide Is Safe and Effective. It Comes with Manageable Side Effects That Decrease with Continued Use.

The Essential Info
The U.S. Food and Drug Administration (FDA) has concluded that benzoyl peroxide is both safe and effective for treating acne.
A quote from the FDA in 2010: “We, the Food and Drug Administration (FDA), are issuing this final rule to include benzoyl peroxide as a generally recognized as safe and effective (GRASE) active ingredient in over-the-counter (OTC) topical acne drug products.”
However, benzoyl peroxide commonly causes side effects such as skin dryness and irritation. These side effects are most predominant during the first few weeks of treatment, and usually lessen dramatically after about one month of treatment. To minimize side effects, start with a pea-size amount of benzoyl peroxide every other day and moisturize your skin after using benzoyl peroxide. Apply sunscreen after benzoyl peroxide if you plan to spend time outside.

The Science
- The History of Verdicts on Benzoyl Peroxide’s Safety
- What Warnings Does the FDA Require on Benzoyl Peroxide Product Labels?
- It May Be Safe to Use Benzoyl Peroxide During Pregnancy and Breastfeeding
- Side Effects of Benzoyl Peroxide
- How to Minimize the Side Effects of Benzoyl Peroxide
Benzoyl peroxide is available both as an over-the-counter and prescription medication for treating acne, in 2.5% to 10% strength. It is inexpensive, easy to apply, and most importantly, effective.1-3
However, some users wonder about the safety of using benzoyl peroxide on a regular basis. As we will see, benzoyl peroxide is in fact safe.
The U.S. Food and Drug Administration (FDA) is the regulatory agency that decides whether a particular medicinal ingredient is safe for the American public based on research evidence. In its latest ruling, the FDA concluded that benzoyl peroxide is both safe and effective as a topical acne treatment. On March 4, 2010, the FDA published this statement:
“We, the Food and Drug Administration (FDA), are issuing this final rule to include benzoyl peroxide as a generally recognized as safe and effective (GRASE) active ingredient in over-the-counter (OTC) topical acne drug products. In addition, this final rule includes new warnings and directions required for OTC acne drug products containing benzoyl peroxide.”4
Let’s take a look at what caused the initial concerns about benzoyl peroxide’s safety, and why the FDA has put those concerns to rest.
The History of Verdicts on Benzoyl Peroxide’s Safety
The verdict on benzoyl peroxide’s safety changed twice over the years as more research became available:
- 1985: Safe. The FDA originally classified benzoyl peroxide as a Category I or GRASE (Generally Recognized As Safe and Effective) ingredient.
- 1991: Uncertain. The FDA changed the safety classification of benzoyl peroxide to Category III, meaning “more data needed,” based on safety concerns due to side effects seen in some animal studies.
- 2010: Definitely safe. The FDA returned benzoyl peroxide to its original Category I based on new data showing its safety in humans.4
Why did the FDA change the classification of benzoyl peroxide in 1991?
The FDA changed its classification of benzoyl peroxide in 1991 because some preliminary evidence suggested that benzoyl peroxide might be carcinogenic in mice.

The evidence came from a study published in the medical journal Cancer Letters in 1984. This study found that applying benzoyl peroxide to mice for a year caused skin cancer in 90% of the mice.5 However, the researchers used mice who were very prone to developing skin cancer. The scientists also combined benzoyl peroxide with acetone, which is itself damaging to the skin. In other words, the results of this study provide weak evidence that benzoyl peroxide might cause cancer in mice but says nothing about humans.
Expand to reveal details of study
In this study, mice were treated with topical benzoyl peroxide at a concentration of 100 mg/ml for 51 weeks. At the end of the study, 90% of the mice had developed skin tumors.5 However, several reasons make it difficult to interpret the results of the study:
- The mice were Sencar mice, which stands for “SENsitivity to CARcinogenesis.” In other words, these mice were bred to develop skin tumors spontaneously and frequently, so the fact that they developed tumors after treatment with benzoyl peroxide does not mean much.
- The benzoyl peroxide was dissolved in acetone, a chemical that can damage the skin and may have contributed to the tumors. In other words, benzoyl peroxide is not the only suspect that may have caused the cancer.
- The mice who were treated with benzoyl peroxide did not die sooner or have lower body weights compared to mice who received just acetone. In other words, the skin tumors do not seem to have threatened the health of the mice.5
On the other hand, Sencar mice who were treated with acetone by itself did not develop tumors, so this does suggest that benzoyl peroxide had something to do with causing the tumors.5 Taken together, the results are somewhat contradictory and suggest the need for more research.

At the same time, another study found that benzoyl peroxide caused no increase of cancer in mice and might even protect them from skin cancer. This study was published in the Journal of Investigative Dermatology in 1986. The researchers used a gel containing benzoyl peroxide, the same one that humans apply for topical treatment. They found that mice treated with benzoyl peroxide gel did not develop more cancer compared to mice treated with just gel without benzoyl peroxide. Not only that, but benzoyl peroxide gel, or just gel alone without benzoyl peroxide in it, tended to protect mice from getting skin cancer when the mice were exposed to the sun’s rays.6
The results of these two studies were contradictory, and until further evidence became available, the FDA decided to classify benzoyl peroxide as a Category III drug, meaning more data were needed.
Why did the FDA change the classification of benzoyl peroxide again in 2010?
Later, new evidence showed that benzoyl peroxide does not cause cancer and is indeed safe. This is why the FDA changed its classification back to Category I.4
The FDA also ruled that labels for products containing benzoyl peroxide need to include specific warnings to minimize the risk of side effects.
What Warnings Does the FDA Require on Benzoyl Peroxide Product Labels?
The FDA requires all products containing benzoyl peroxide to include the following information on the label:

It May Be Safe to Use Benzoyl Peroxide During Pregnancy and Breastfeeding
When it comes to using benzoyl peroxide during pregnancy, the FDA considers benzoyl peroxide a Category C drug. That means that there is not enough research on how benzoyl peroxide might affect the development of an unborn baby. It also means that animal studies have found some possible risks. On the other hand, when you apply benzoyl peroxide to the skin, very little of it ends up in the blood, so it is unlikely to reach a baby in the uterus and affect its health. On balance, doctors consider benzoyl peroxide safe to use during pregnancy.7,8 However, it’s important to talk to your doctor before using any medication while you are pregnant.
Similarly, if you are breastfeeding, it is likely safe to use benzoyl peroxide on your skin, as long as the baby does not come in contact with the drug.7 Again, talk to your doctor before using any medication while breastfeeding.
Side Effects of Benzoyl Peroxide
Despite being safe, benzoyl peroxide does cause side effects, which include:
- Skin dryness and irritation (burning/stinging)
- Sensitivity to the sun
- Bleaching of clothing, towels, and bed linens
- [Rare] Allergic contact dermatitis (allergic rash)9-11

These side effects often lead acne patients to give up treatment after only a few days of use. According to a 2007 article in the Pharmaceutical Journal, “Unfortunately, benzoyl peroxide-based products are often rejected by patients after a few days’ use, once their skin starts to flake or become inflamed.”1
The side effects of benzoyl peroxide are dose-dependent, which means that a larger amount or higher concentration causes more side effects. Severe side effects, such as allergic contact dermatitis, are rare and occur only in approximately one out of every 500 patients.1,9,10
The side effects of benzoyl peroxide are also manageable and tend to decrease over time as the patient continues to use the product.1
How to Minimize the Side Effects of Benzoyl Peroxide
Here are some tips to help you minimize and manage the side effects when you use benzoyl peroxide:
- Start slowly: Begin treatment with a small amount of benzoyl peroxide, and apply it only every other day if you are experiencing side effects.
- Use a moisturizer: Any topical benzoyl peroxide formulation will tend to cause skin dryness and irritation, so using a moisturizer at the same time can help to minimize these side effects.
- Apply sunscreen to go outside: Since benzoyl peroxide might make the skin more sensitive to the sun, apply sunscreen after treatment if you intend to go outside.
- Stick with it: The side effects of benzoyl peroxide get better with time, so it is worthwhile to stick with the treatment.1
How Benzoyl Peroxide Works
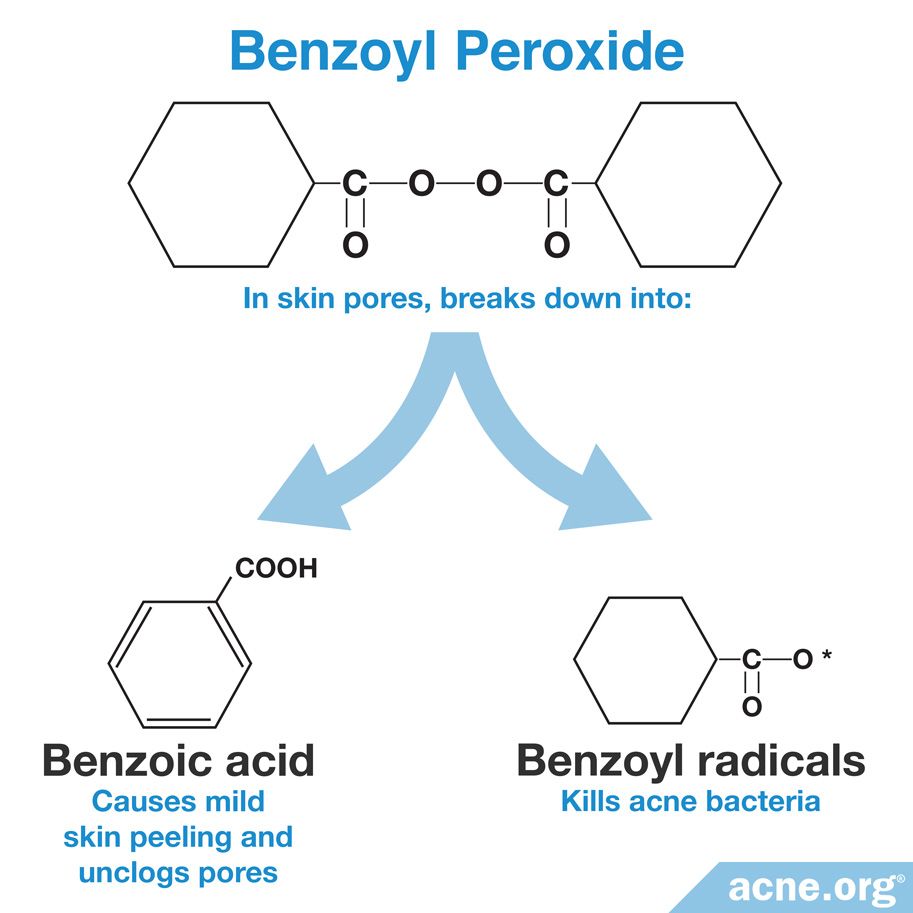
Pure benzoyl peroxide comes in the form of white crystals that do not dissolve in water, but do dissolve well in fats. This allows benzoyl peroxide to easily pass through the outermost layer of the skin and enter skin pores.1
Once benzoyl peroxide gets into skin pores, it breaks down into two components: benzoyl radicals and benzoic acid. The first of these kill acne bacteria, and the second works to unclog pores. Together, these two activities make benzoyl peroxide effective against acne but also cause mild side effects.1
The History of Benzoyl Peroxide Use
Benzoyl peroxide first came into use as an agricultural and industrial chemical. In 1929, researchers first wrote about the possible value of treating wounds and burns with benzoyl peroxide. It was only in the 1960s that doctors began to use benzoyl peroxide as an acne remedy. Now, however, benzoyl peroxide is a popular acne treatment, either alone or in combination with other treatments.1
References
- Tucker, R. & Walton, S. The role of benzoyl peroxide in the new treatment paradigm for acne. Pharm. J. 279, 48 – 53 (2007). https://www.ncbi.nlm.nih.gov/pubmed/23839205
- Sagransky, M., Yentzer, B. A. & Feldman, S. R. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin. Pharmacother. 10, 2555 – 2562 (2009). https://www.ncbi.nlm.nih.gov/pubmed/19761357
- Mohammad, T. F. Acne therapeutics: A closer look at Benzoyl Peroxide. Skinmed 13, 94 – 96 (2015). https://www.ncbi.nlm.nih.gov/pubmed/26137733
- Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format; Topical acne drug products for over-the-counter human use; Final rule. (2010, March 4). Retrieved from https://www.federalregister.gov/documents/2010/03/04/2010-4424/classification-of-benzoyl-peroxide-as-safe-and-effective-and-revision-of-labeling-to-drug-facts
- Kurokawa, Y., Takamura, N., Matsushima, Y., Imazawa, T. & Hayashi, Y. Studies on the promoting and complete carcinogenic activities of some oxidizing chemicals in skin carcinogenesis. Cancer Lett. 24, 299 – 304 (1984). https://www.ncbi.nlm.nih.gov/pubmed/6437666
- Iversen, O. H. Carcinogenesis studies with benzoyl peroxide (Panoxyl gel 5%). J. Invest. Dermatol. 86, 442 – 448 (1986). https://www.ncbi.nlm.nih.gov/pubmed/3091706
- Matin, T. & Goodman, M. B. Benzoyl Peroxide. [Updated 2019 Sep 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537220/
- Chien, A. L., Qi, J., Rainer, B., Sachs, D. L. & Helfrich, Y. R. Treatment of acne in pregnancy. J. Am. Board Fam. Med. 29, 254-262 (2016). https://pubmed.ncbi.nlm.nih.gov/26957383/
- Topical acne products for over-the-counter human use – revision of labeling and classification of benzoyl peroxide as safe and effective. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research 1 – 8 (2011). doi:10.1186/1477-7525-4-79 https://www.fda.gov/media/80442/download
- Tripathi, S. V, Gustafson, C. J., Huang, K. E. & Feldman, S. R. Side effects of common acne treatments. Expert Opin. Drug Saf. 12, 39 – 51 (2013). https://www.ncbi.nlm.nih.gov/pubmed/2316333
- Kawashima, M., Hashimoto, H., Alio Sáenz, A. B., Ono, M. & Yamada, M. Is benzoyl peroxide 3% topical gel effective and safe in the treatment of acne vulgaris in Japanese patients? A multicenter, randomized, double-blind, vehicle-controlled, parallel-group study. J. Dermatol. 41, 795-801 (2014). https://pubmed.ncbi.nlm.nih.gov/25132461/
 Acne.org Products
Acne.org Products