Isotretinoin Causes Severe Birth Defects and Miscarriage

The Essential Info
EXTREME CAUTION: Isotretinoin (Accutane®) interferes with fetal development and causes severe and life-threatening birth defects. In the fetus, isotretinoin disrupts normal brain development, hinders heart and immune system development, can deform the ears/face/head, and can result in death of the unborn child.
In the mother, isotretinoin might increase the chance of developing inflammatory bowel disease (IBD) as well as experiencing depressive and suicidal thoughts.
Therefore, it is imperative that women who are pregnant or planning to become pregnant DO NOT TAKE ISOTRETINOIN.

The Science
Isotretinoin (Accutane®) is a medication that dermatologists sometimes prescribe to treat severe acne. Although effective, isotretinoin can cause serious and dangerous side effects to the fetus during pregnancy, and thus must be avoided if a female patient is pregnant or may become pregnant.
Any medication that can cause the malformation of an embryo is called a teratogen. Of all available prescriptions for any disease, isotretinoin is the most common teratogen on the market. It can interfere with normal fetal development and cause severe and life-threatening birth defects.1
Unfortunately, one scientific survey found that many teen girls and women with acne are not aware of the serious teratogenic potential of isotretinoin.2 If you are reading this, you are already ahead of the game.
Isotretinoin Side Effects on the Fetus
Due to the teratogenic effects of isotretinoin, it would be unethical for scientists to perform a typical scientific study investigating the effect of isotretinoin on pregnant women. Therefore, scientists studying the link between isotretinoin and birth defects have relied on studies that follow women who begin taking isotretinoin and happen to become pregnant during their treatment course, as well as studies that use animal models.
All the studies agree that exposure to isotretinoin during pregnancy causes miscarriage, birth defects, and infant deaths.3-7
Expand to read details of studies

A 1984 report by the Centers for Disease Control followed two groups of pregnant women that had been exposed to isotretinoin. In the first group of 18 women, there were 13 miscarriages, 4 infants without malformations, and one infant with severe malformations. In the second group of 16 women, there were 6 spontaneous abortions, 9 severe developmental abnormalities, and 1 infant without malformations. The researchers concluded that isotretinoin exposure during pregnancy is significantly associated with an increased risk of birth defects or fetal and infant death.3

Two studies published in the 1990s found that when chicken embryos are exposed to isotretinoin, it causes severe brain and heart defects. Specifically, the researchers found that isotretinoin severely affects the shape and growth of cells called neural crest cells. Neural crest cells are important developmental cells that eventually will create the skin, facial bone and cartilage, certain muscles, and nervous system cells. Therefore, when isotretinoin disrupts the normal growth and shape of these cells, it can result in defects in numerous different regions of the body.4,5

A large study published in BMJ Open looked at 200,000 pregnancies in the Netherlands between 1999 and 2007. This study found that 51 pregnant women were exposed to isotretinoin during pregnancy or within 30 days before conception. Of these 51, 3 resulted in infant deaths, and 2 resulted in serious infant birth defects. However, this data is not complete as it did not report the number of women who had a miscarriage or planned abortion.6

A 2007 study published in the British Journal of Clinical Pharmacology followed 14 pregnant women who had been exposed to isotretinoin in order to record the effects on their children. During the course of the pregnancy there were 3 miscarriages, 2 infant deaths upon delivery, 1 infant presenting with severe facial defects, and 8 infants without malformations.7
When it comes to women who were exposed to isotretinoin long before becoming pregnant, the research is more reassuring. One large study found that women who took isotretinoin over a year before becoming pregnant were no more likely to have babies with birth defects than women who never took isotretinoin at all.8 Therefore, if you do take isotretinoin, it is important to stop taking it well before you try to conceive.
Expand to read details of study

A 2022 study published in the journal Children looked at the effects of exposure to isotretinoin 1-2 years before becoming pregnant. The study included 322 women who took isotretinoin, on average, 18 months before becoming pregnant, as well as 1137 women who never took isotretinoin prior to becoming pregnant. A total of 1.2% of babies born to mothers who had taken isotretinoin exhibited birth defects, but these birth defects were not the kind expected from taking isotretinoin. In fact, there were more birth defects among babies born to women who never took isotretinoin.8 In other words, taking isotretinoin more than a year before becoming pregnant did not seem to increase the risk of birth defects.
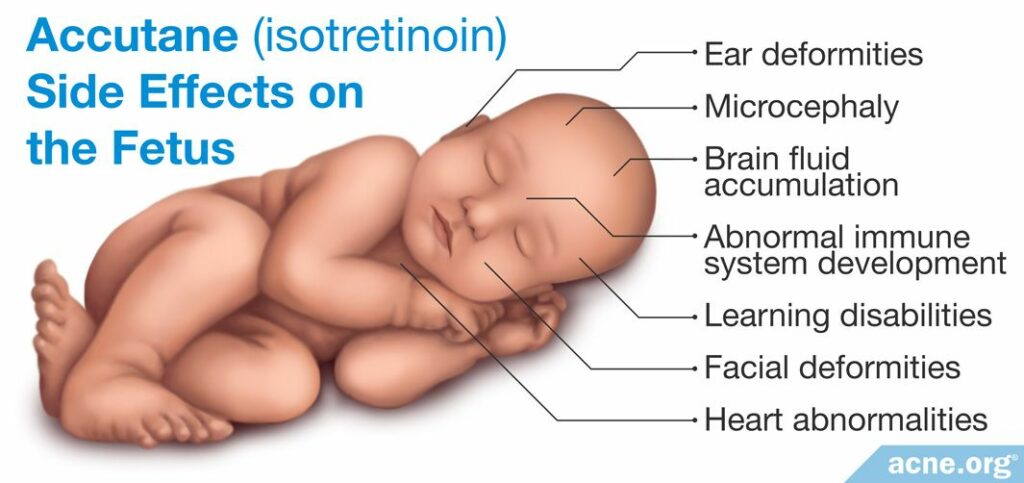
Children born to mothers exposed to isotretinoin during pregnancy often display a condition known as “isotretinoin embryopathy.” This disorder is associated with a variety of symptoms that include:
- Ear deformities (small ear, no ears, deformed ear canals)
- Facial deformities (small jaw, widely spaced eyes, depressed nasal cavity)
- Microcephaly (abnormally small head)
- Heart abnormalities
- Brain Fluid Accumulation
- Learning disabilities (low IQ)
- Abnormal immune system development
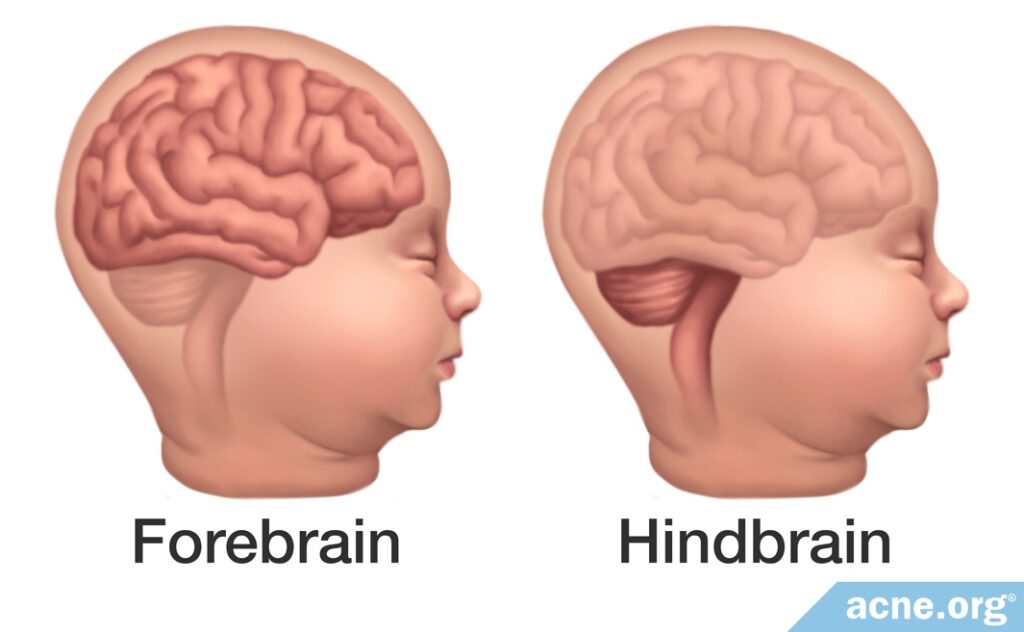
Children with these abnormalities are divided into two groups based on which part of the brain has been affected:
- The first group has developmental defects in the lower part of the brain, called the hindbrain. This section of the brain is crucial for basic physiological functions like heartbeat and breathing. When affected by isotretinoin, defects in this region often result in miscarriage.
- The second group has developmental defects in the front of the brain, called the forebrain. This section of the brain is crucial for higher cognitive function. When affected by isotretinoin, defects in this region often result in learning disabilities and other cognitive impairments. Children in this group may or may not display any physical malformations.1
Isotretinoin Side Effects on the Mother
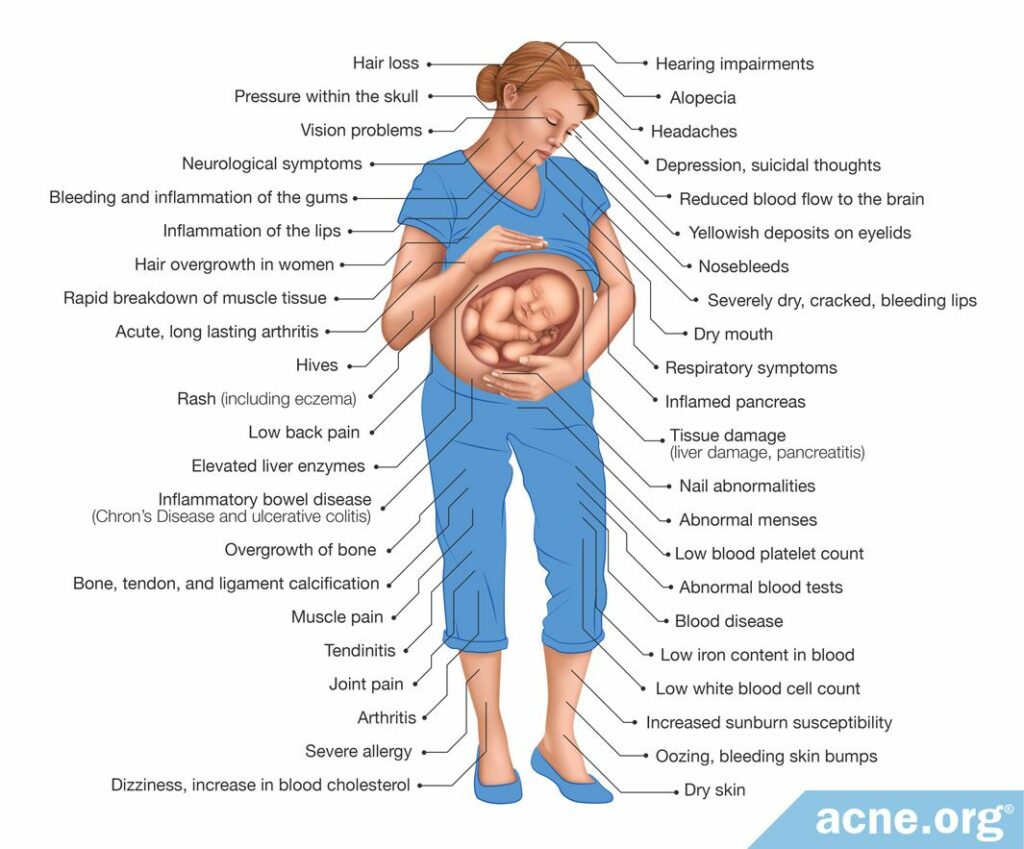
Researchers studying the effects of isotretinoin on non-pregnant women have found common side effects, including:
- Depression & suicidal thoughts9
- Inflammatory bowel disease (Crohn’s disease and ulcerative colitis)9
- Tissue damage (liver damage, pancreatitis)10
- Less serious side effects (dizziness, dry skin, joint pain, headaches, increase in blood cholesterol)10
Because isotretinoin is an oral medication, it affects the entire body. Pregnant women may experience the same side effects as non-pregnant women. However, pregnancy can often worsen depression, suicidal thoughts, and inflammatory bowel disease.11-13
Below are some potential side effects of isotretinoin in women:
The seriousness of the problem with birth defects means that women of child bearing age taking isotretinoin are required to register for the iPLEDGE program. The iPLEDGE program requires that women taking isotretinoin undergo frequent pregnancy tests and commit to using two (2) forms of birth control in order to prevent themselves from getting pregnant.15
Due to the severity of the side effects, pregnancy prevention is absolutely critical. However, if a woman becomes pregnant while taking isotretinoin, she often elects to terminate the pregnancy.8,14 Even though pregnancy prevention programs like iPLEDGE and SMART (an older version of iPLEDGE) have been slightly successful in preventing isotretinoin-related birth defects, studies have found that neither patients nor doctors are fully adherent to these programs, and therefore more effort is needed to minimize the risks.16-19
The Bottom Line
It can’t be stated strongly enough: If you are female and considering isotretinoin, do anything and everything in your power not to become pregnant. And if there is any chance whatsoever that you may become pregnant while on isotretinoin, do not start taking it.
References
- McCaffery, P. J., Adams, J., Maden, M. & Rosa-Molinar, E. Too much of a good thing: retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. Eur. J. Neurosci. 18, 457-472 (2003). https://www.ncbi.nlm.nih.gov/pubmed/12911743
- Alshaalan, Z. M. Knowledge on the use of isotretinoin and its side effects and awareness towards Saudi FDA-Pregnancy Prevention Program among the female acne patients: A Northern Saudi study. Medicina (Kaunas) 58, 1609-1620 (2022). https://pubmed.ncbi.nlm.nih.gov/36363566/
- Centers for Disease Control and Prevention. Epidemiologic Notes and Reports Isotretinoin — A Newly Recognized Human Teratogen. Morbidity and Mortality Weekly Report. 33, 171-173 (1986). https://www.cdc.gov/mmwr/preview/mmwrhtml/00000310.htm
- Orris, A. S., Banicky, L. C., & Wiens, D. J. Isotretinoin alters morphology, polarity, and motility of neural crest cells in culture. Reprod. Toxicol. 13, 45-52 (1999). https://www.ncbi.nlm.nih.gov/pubmed/10080299
- Wiens DJ, Mann, T. K., Fedderson, D. E., Rathmell, W. K. & Franck, B. H. Early heart development in the chick embryo: effects of isotretinoin on cell proliferation, alpha-actin synthesis, and development of contractions. Differentiation. 51,105-112 (1992). https://www.ncbi.nlm.nih.gov/pubmed/1473624
- Zomerdijk, I. M. et al. Isotretinoin exposure during pregnancy: a population-based study in The Netherlands. BMJ Open. 4, e005602 (2014). https://www.ncbi.nlm.nih.gov/pubmed/25392022
- Berard A, et al. Isotretinoin, pregnancies, abortions and birth defects: a population-based perspective. Br. J. Clin. Pharmacol. 63, 196-205 (2007). https://www.ncbi.nlm.nih.gov/pubmed/17214828
- Brzezinski, P., Zonda, G. I., Hincu, M. A., Vasilache, I. A., Chiriac, A., Ciuhodaru, M. I., Borowska, K. & Paduraru, L. A multicenter cohort study evaluating the teratogenic effects of isotretinoin on neonates. Children (Basel) 9, 1-6 (2022). https://pubmed.ncbi.nlm.nih.gov/36360340/
- Tan, J., Boyal, S., Desai, K. & Knezevic, S. Oral isotretinoin: New developments relevant to clinical practice. Dermatol. Clin. 34, 175-184 (2016). https://www.ncbi.nlm.nih.gov/pubmed/27015777
- Charakida, A., Mouser, P. E. & Chu, A. C. Safety and side effects of the acne drug, oral isotretinoin. Expert Opin. Drug Saf. 3, 119-129 (2004). https://www.ncbi.nlm.nih.gov/pubmed/15006718
- Shand, A. W., Chen, J. S., Selby, W., Solomon, M. & Roberts, C. L Inflammatory bowel disease in pregnancy: a population-based study of prevalence and pregnancy outcomes. BJOG 123, 1862-1870 (2016). https://www.ncbi.nlm.nih.gov/pubmed/26924786
- Grigoriadis, S. et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J. Clin. Psychiatry. 74, e321-341 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23656857
- Stein, A. et al. Effects of perinatal mental disorders on the fetus and child. Lancet 384, 1800-1819 (2014). https://www.ncbi.nlm.nih.gov/pubmed/25455250
- Paidisetty, P. S., Wang, L. K., Zamil, D. H., Fu, S. & Nawas, Z. Y. Isotretinoin, vitamin A supplements, and unintended pregnancies in post Roe v. Wade America. Cureus 14, e31442 (2022). https://pubmed.ncbi.nlm.nih.gov/36523684/
- Pierson, J. C., Ferris, L. & Schwarz, E. B. We pledge to change iPLEDGE. JAMA Dermatol. 151, 701-702 (2015). https://www.ncbi.nlm.nih.gov/pubmed/25853235
- Tan, J. K. & Shear, N. Oral isotretinoin: ensuring safe use while not limiting access to those who need it. CMAJ. 189, E510 (2017). https://pubmed.ncbi.nlm.nih.gov/28385900/
- Choi, J. S., Koren, G. & Nulman, I. Pregnancy and isotretinoin therapy. CMAJ. 185, 411-413 (2013). https://pubmed.ncbi.nlm.nih.gov/23296582/
- Henry, D., Dormuth, C., Winquist, B. et al. Occurrence of pregnancy and pregnancy outcomes during isotretinoin therapy. CMAJ. 188, 723-730 (2016). https://pubmed.ncbi.nlm.nih.gov/27114489/
- Khiali, S., Gharekhani, A. & Entezari-Maleki, T. Isotretinoin; A review on the utilization pattern in pregnancy. Adv. Pharm. Bull. 8, 377-382 (2018). https://pubmed.ncbi.nlm.nih.gov/30276133/
 Acne.org Products
Acne.org Products