Comedogenic Ingredients Are Ingredients That Clog Pores. There Are 45 Ingredients to Watch Out For.
- What Does Comedogenicity Mean?
- How Do Scientists Measure Comedogenicity?
- The 11 Studies Performed Thus Far on Comedogenicity
- Choosing a Non-comedogenic Product for Acne-prone Skin
This article will go in depth and cover everything there is to know about comedogenicity and comedogenic ingredients. For a brief, summarized version of this article, see the shorter, simplified article here.
What Does Comedogenicity Mean?
Clogged pores lead to acne. Comedogenicity is a term that describes the potential of a substance to cause a comedone, which is the scientific name for a clogged pore.
When choosing a cosmetic product for use on acne-prone skin, it is important to consider if its ingredients are comedogenic. Researchers have performed 11 studies investigating the capability of numerous ingredients and final products to clog pores, and this gives us a fair idea of which ingredients to watch out for.
How Do Scientists Measure Comedogenicity?
To determine the comedogenicity of a cosmetic ingredient, scientists use one of two methods:
- Rabbit ear assay
- Human method
Rabbit ear assay [Note: Acne.org does not test on animals]
Scientists perform the rabbit ear assay (REA) by applying a cosmetic ingredient to the skin of the internal surface of a live male albino rabbit ear. Researchers use rabbits to test for comedogenicity because rabbit ear skin is similar to human skin and develops comedones. Comedone formation occurs much faster in rabbits, and this is beneficial to studies because comedogenicity can be determined faster when using the REA than would be possible on human skin.
To perform the REA correctly, several factors must be considered:
- Rabbit gender: Albino male rabbits are used for the REA because their skin pores are larger than those of female rabbits.
- Rabbit age: The rabbits must be 12 weeks old because rabbits less than 12 weeks of age are less likely to develop comedones.
- Number of rabbits: For each substance tested, the REA must be performed on at least three (3) rabbits.
- Location of application: To perform the REA, the substance is applied to a 2×2 centimeter area of the internal ear.
- Amount of substance tested: Researchers must apply at least 0.25 milliliters of cream or ointment or at least 0.1 milliliters of lotion to the rabbit ear. As a general rule, ingredients are tested at 10% strength, and are placed into an inert carrier like propylene glycol or glycerin.
- Negative control: The substance being tested should be applied to one ear, while a control solution that does not contain the substance should be applied to the other ear so a clear comparison can be drawn.
- Duration of testing: The tested substance is applied five days a week for three weeks and not washed off the rabbit’s skin during that period. Comedone formation is examined after two to three weeks.1-4

Evaluating the results of a rabbit ear assay (REA)
After the two- to three-week testing period, researchers examine the number of comedones on the skin. There are three methods scientists use to examine the number of comedones:
- A visual inspection is performed by looking at the rabbit’s ear and counting the number of comedones visible to the naked eye.
- A whole mount is performed by taking a skin sample from the rabbit’s ear and viewing it under the microscope in order to count the number of comedones.
- A histological examination is performed by taking a skin sample from the rabbit’s ear, treating it with special chemicals that provide color to specific regions of the comedone, and then viewing it under the microscope in order to count the number of comedones.
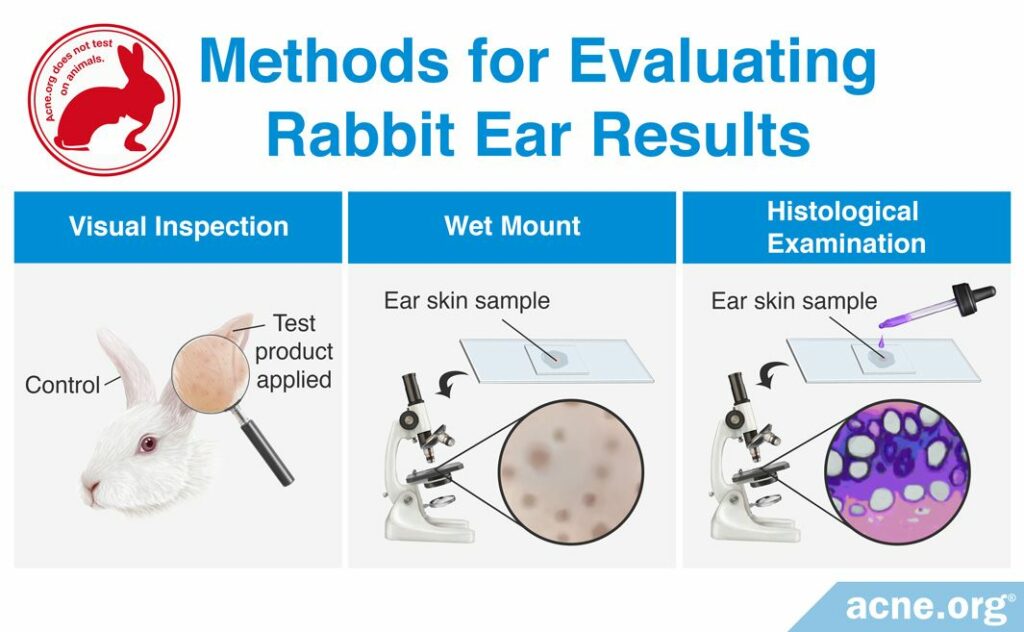
The visual inspection and whole mount methods are easiest to perform rapidly and can be used to identify a strong comedogenic response. However, they are not effective at identifying weak comedogenic responses. The histological examination method is the most reliable and can detect weak comedogenicity, but is a more difficult method and requires more time. Whichever method is used, researchers report on the comedogenicity of a cosmetic ingredient with a scale. The American Academy of Dermatology recommends using a scale of 0 – 3, with 0 representing non-comedogenic products and 3 representing highly comedogenic products. However, a few research groups choose to report their REA findings using scales from 0 – 4 or 0 – 5.1-4
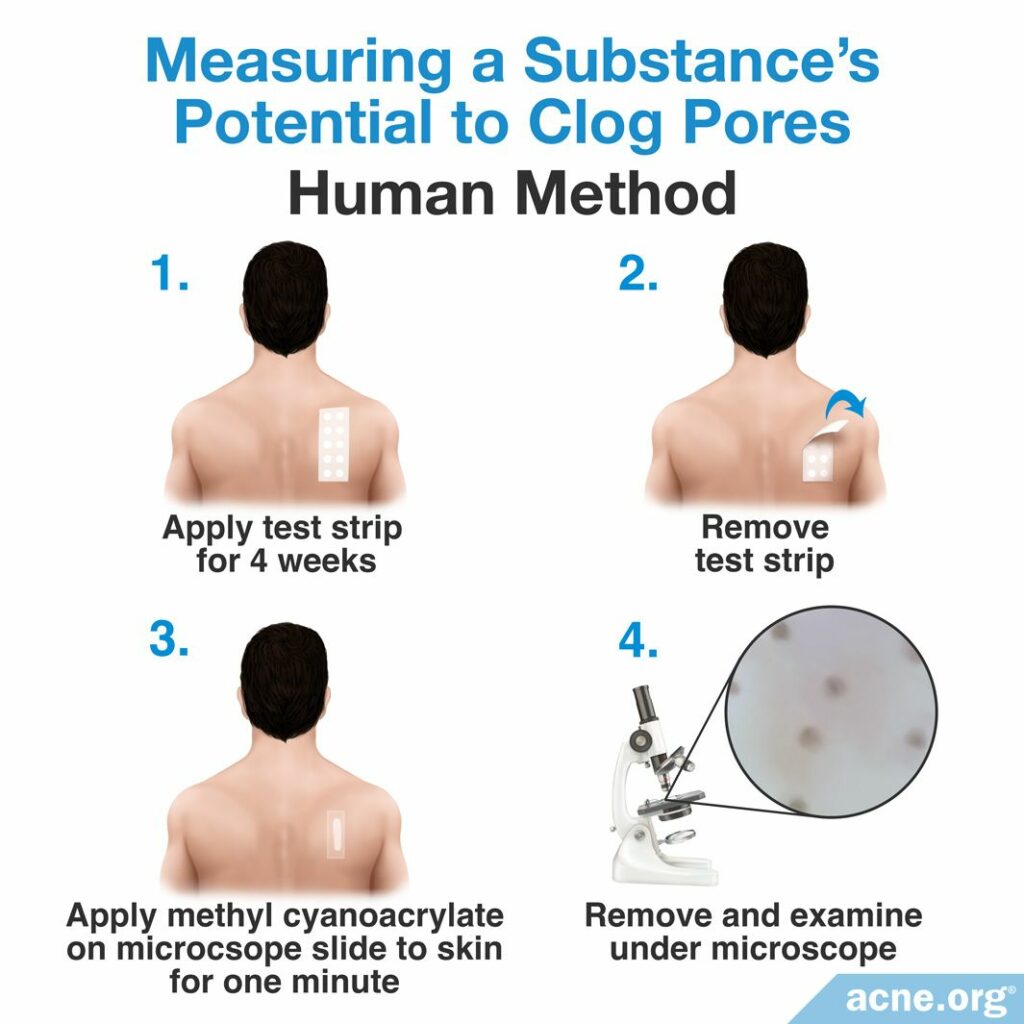
Human method
Scientists perform the human method by applying a cosmetic ingredient onto the upper back of people with naturally large skin pores daily for at least four weeks. Then, the comedogenicity of the tested substance is examined through a process called cyanoacrylate follicular biopsy. To perform a cyanoacrylate follicular biopsy, the researchers coat the upper back of study-participants with a thin layer of a special glue called methyl cyanoacrylate. On top of the glue is placed a glass slide. After resting the slide on the glue for one minute, the researchers remove it from the upper back. When the slide is removed, the glue sticks to and removes the uppermost layer of the skin. Viewing this skin under a microscope allows for the scientists to examine the number and content of comedones in the uppermost layer of the skin. Like the REA, scientists performing the human method of comedogenicity testing rate the comedone forming ability of the ingredient also with a 0 – 3, 0 – 4, or 0 – 5 scale.3,4
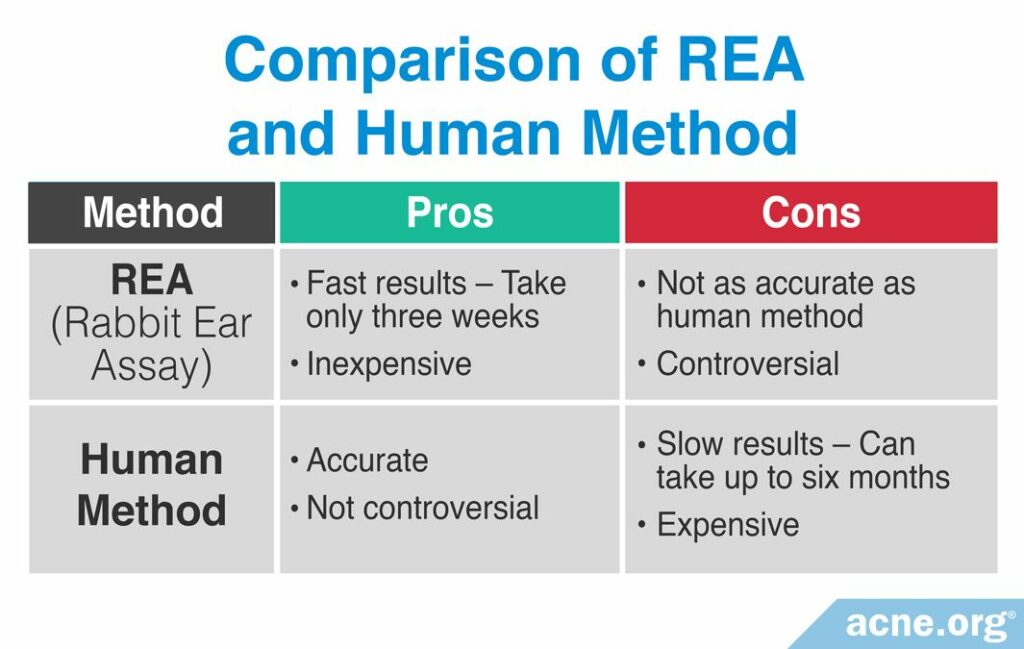
Comparing the REA and human method
There are benefits and drawbacks to using either the REA or human method for determining comedogenicity. For example, because rabbit ear skin is more sensitive than human skin, substances clog pores more quickly in rabbit skin. Therefore, the REA method can produce results within two to three weeks, while the human method can take up to six months in some cases. However, not all substances that clog pores in rabbits clog pores also in humans, so the human method is a more accurate method at determining what clogs pores in humans. The following table displays a comparison of the pros and cons of using either the REA or the human method.
To determine if a substance that clogs pores in rabbits will also clog pores in humans, scientists compared the data from several studies and drew two conclusions.4-6
- If the comedogenicity of a substance is 0 – 2 in the REA, then it is unlikely to clog pores in humans.
- If the comedogenicity of a substance is greater than 2 in the REA, the experiment should be repeated using the human method to determine if it clogs pores in humans.
Despite the differences in the two methods, research commonly is performed using both.

The 11 Studies Performed Thus Far on Comedogenicity
Scientists have performed 11 studies investigating the comedogenicity of cosmetic ingredients or of cosmetic products using either the REA or human method, or both. Let’s take a look at them one by one.
Study #1


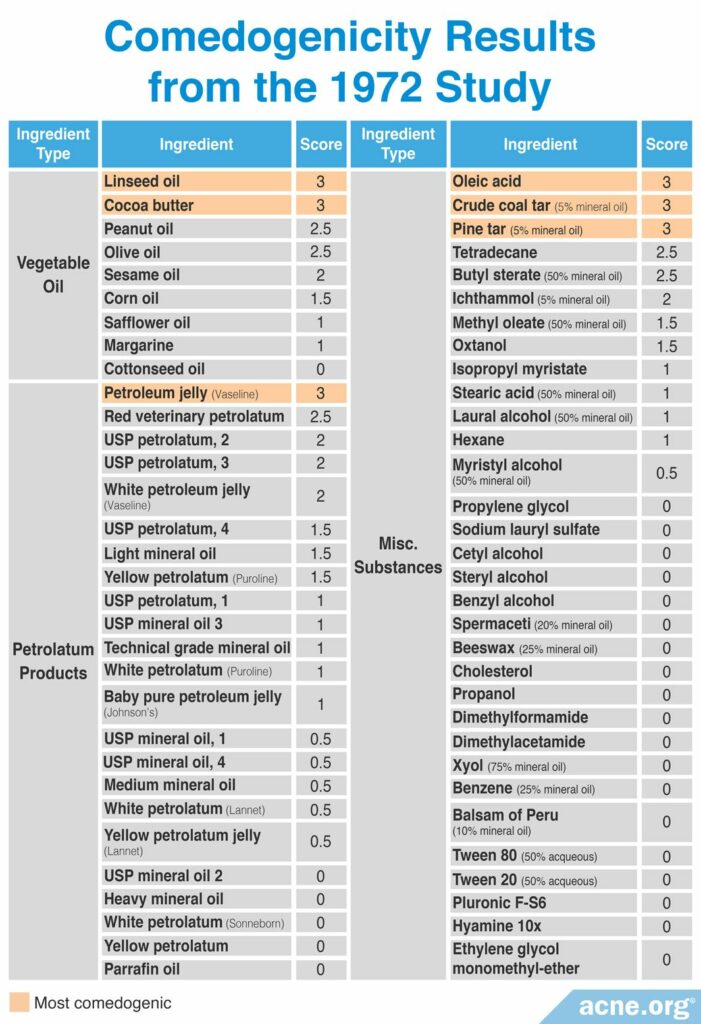
A 1972 study published in the Archives of Dermatology first tested the comedogenicity of cosmetic ingredients using the REA and human method with a scale of 0 – 3. Comedogenicity was determined using the whole mount method. The products investigated in this study included 7 types of vegetable oil, 23 varieties of petrolatum products, such as petroleum jelly, and several miscellaneous ingredients and consumer products, including five brands of soap. The researchers found that linseed oil, cocoa butter, and olive oil were the most comedogenic oils; Vaseline® petroleum jelly was the most comedogenic petrolatum product; acetylated lanolin alcohols were the most comedogenic lanolins, and the all soaps were non-comedogenic. The table below displays the full ingredient list tested in this study with their associated REA scores. The study did not report the human method scores. However, the researchers did note that human skin was less sensitive to the ingredients than rabbit skin. Specifically, they found that only ingredients with a grade of 2 or 3 by the REA were comedogenic in humans.7
Study #2


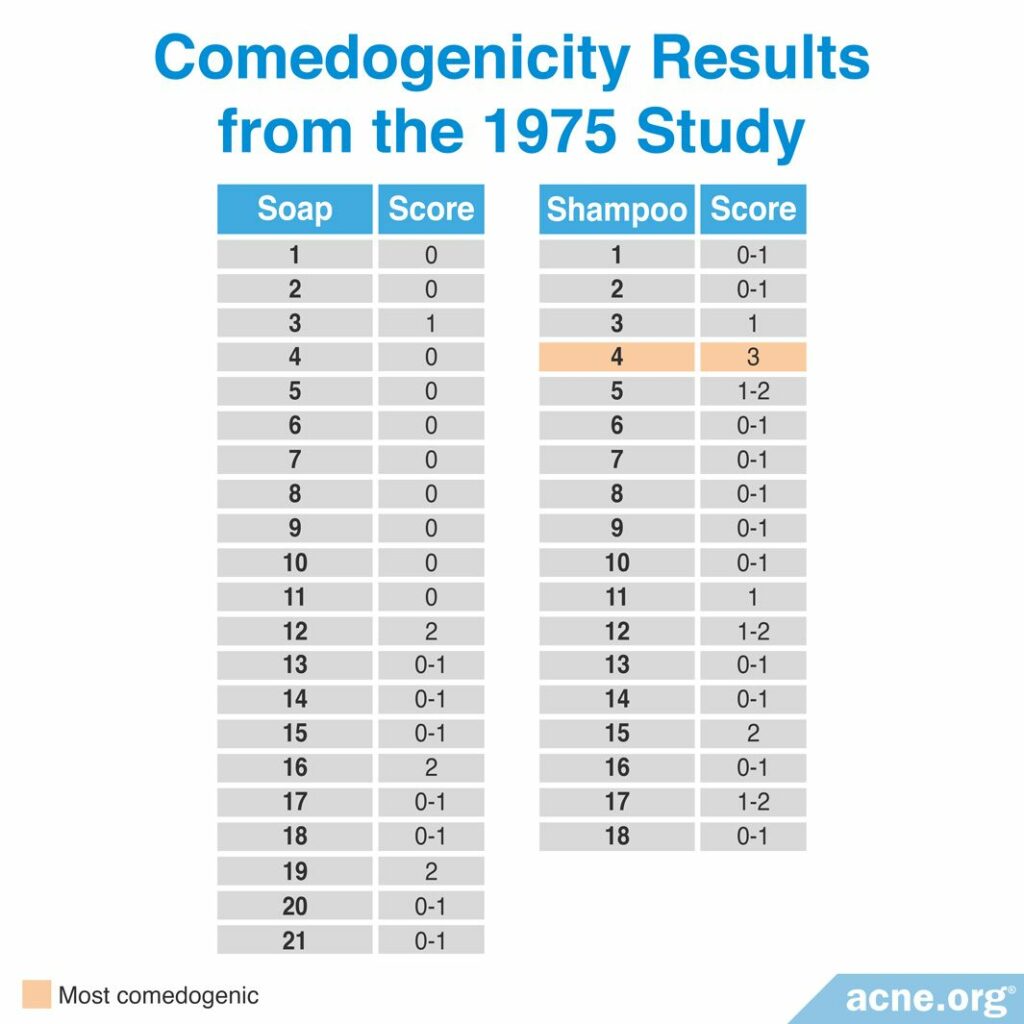
A 1975 study published in the Archives of Dermatology investigated the comedogenicity of 21 soaps and 18 shampoos using the REA with a scale of 0 – 3. Comedogenicity was determined using the whole mount and histological examination methods. They found that all the soaps and all but one of the shampoos were non-comedogenic. However, many of the soaps and shampoos caused skin irritation. The table below shows the full comedogenicity results. The study did not provide the brand of the soaps or shampoos they tested and instead labeled them 1 – 21 for soaps and 1 – 18 for shampoos.8
Study #3


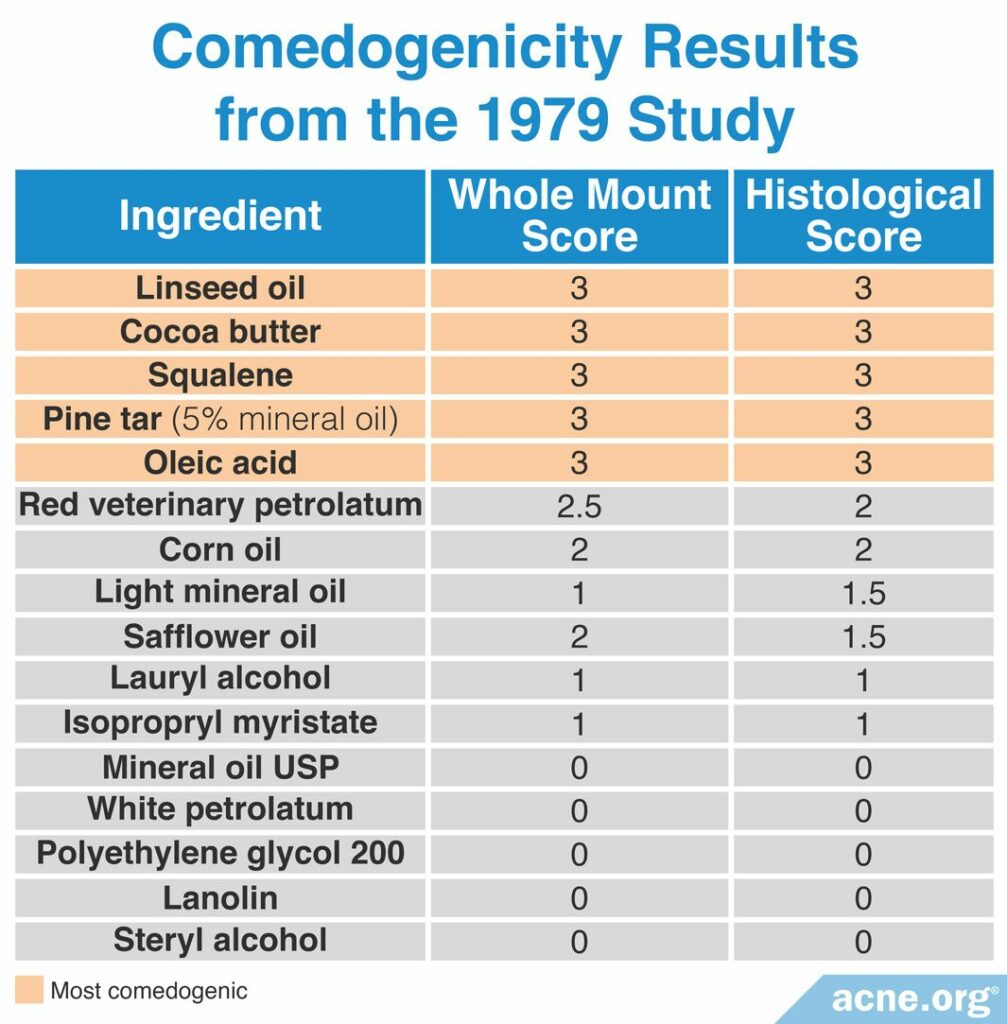
A 1979 study published in the British Journal of Dermatology examined the comedogenicity of 16 cosmetic ingredients using the REA with a scale of 0 – 3. The comedogenicity of the ingredients was measured using both the whole mount method and the histological method. The most comedogenic ingredients were linseed oil, cocoa butter, squalene, pine tar, and oleic acid, among others. The table below shows the comedogenicity scores for all 16 products.2
Study #4


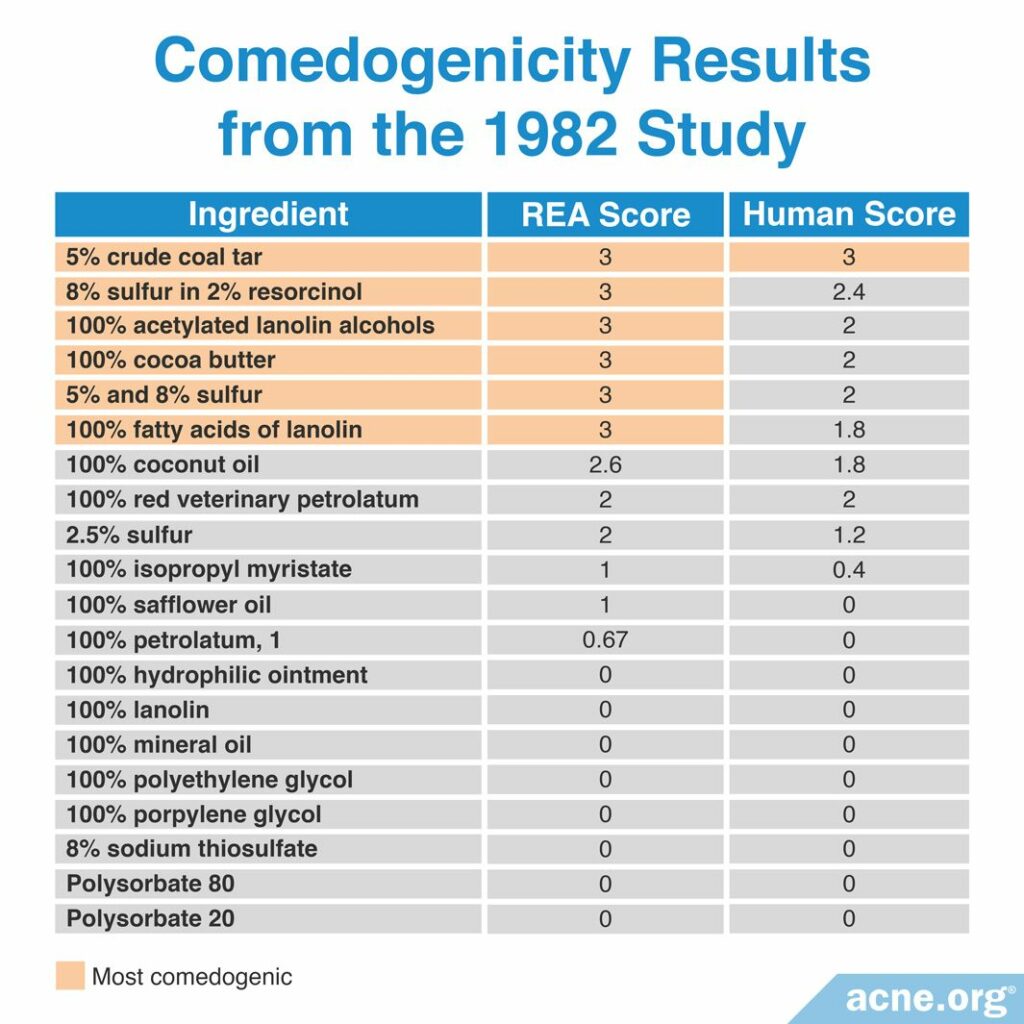
A 1982 study published in the Archives of Dermatology examined the comedogenicity of several cosmetic ingredients and cosmetic products using the REA and human method with a scale of 0 – 3. Comedogenicity was determined using the histological examination method. The researchers found that the most comedogenic ingredients were 5% crude coal tar, sulfur products, acetylated lanolin alcohols, cocoa butter, and lanolin fatty acids. They found also that between the REA and human method, the human method was less sensitive. The table below displays the comedogenicity scores for the ingredients and products tested in this study.9
Study #5


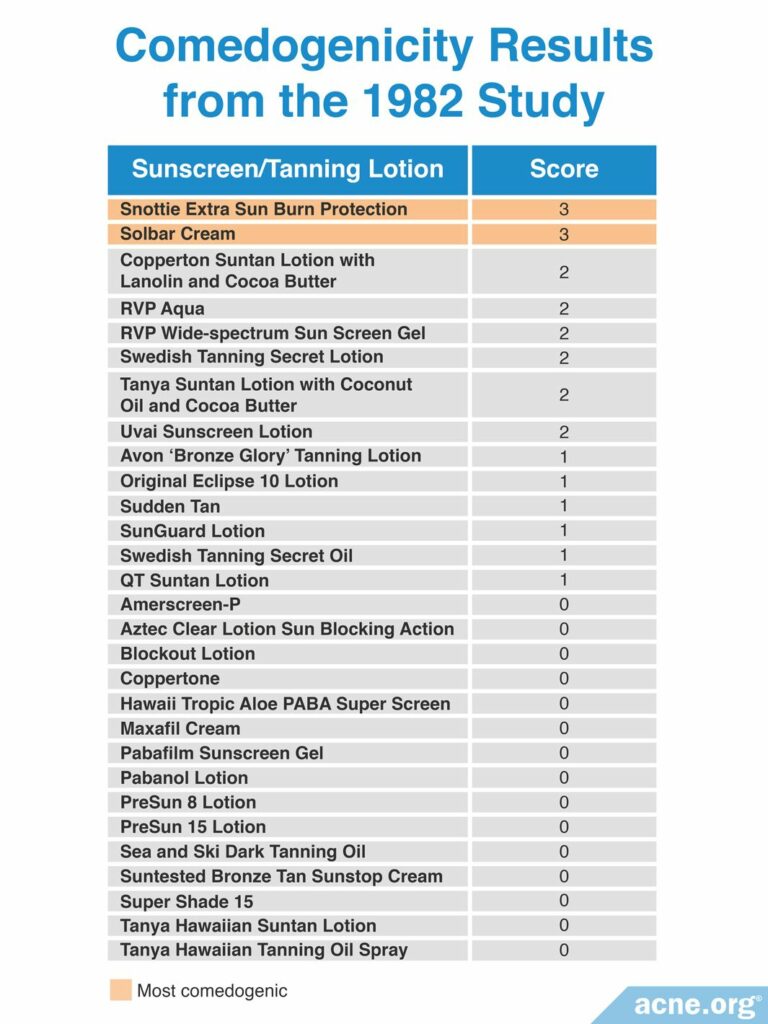
Another 1982 study published in the Archives of Dermatology examined the comedogenicity of 29 sunscreen products using the REA with a scale of 0 – 3. Comedogenicity was determined using the whole mount method. The researchers found that 14 of the 29 sunscreens were comedogenic, represented by a score of at least 1. In a follow-up experiment they retested the specific sunscreen ingredients by themselves and found that the ingredients responsible for protecting the skin from harmful sun rays were non-comedogenic. Therefore, the comedogenicity of sunscreens may have been due to the inactive ingredients. They used REA only for other ingredients in sunscreens and not for the ingredients that protect from UV light. The table below shows comedogenicity scores for the 29 tested sunscreen products.10
Study #6


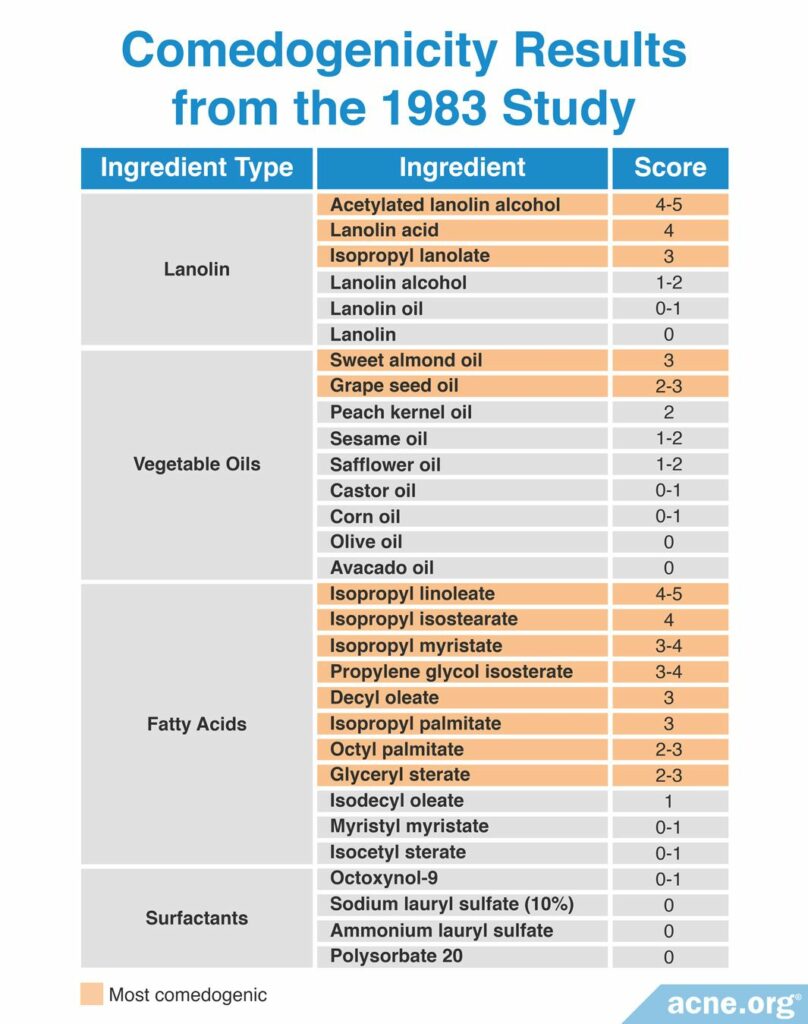
A 1983 study published in the Journal of the Society of Cosmetic Chemists examined the comedogenicity of several cosmetic ingredients using the REA with a scale of 0 – 5. Comedogenicity was determined using the histological examination method. The researchers found that the most comedogenic ingredients were acetylated lanolin alcohol, lanolin acid, isopropyl linoleate, and isopropyl isostearate. The table below shows the comedogenicity scores for all tested ingredients.11
Study #7


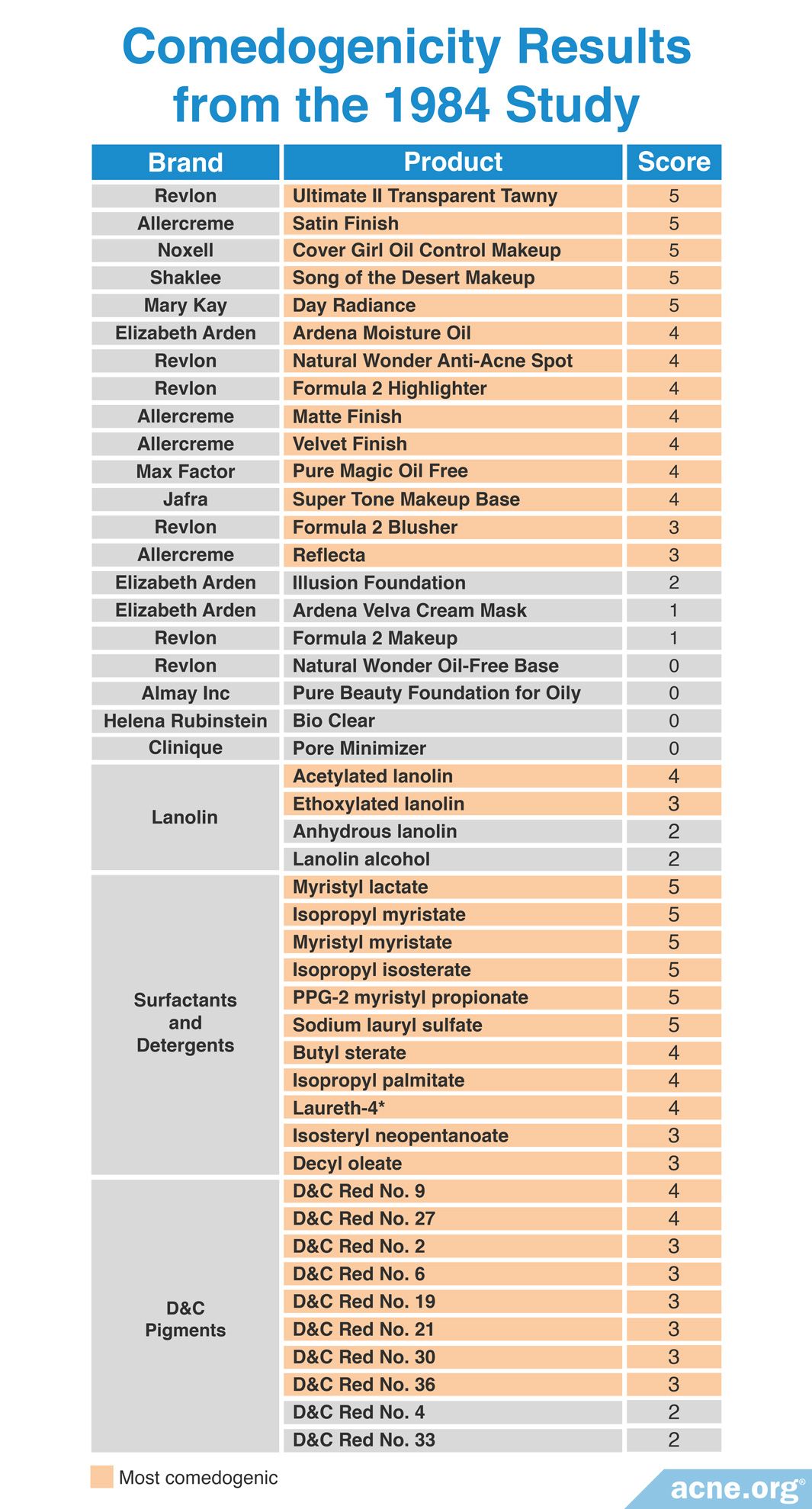
A 1984 study published in the Journal of the American Academy of Dermatology examined the comedogenicity of 21 cosmetic products and 25 cosmetic ingredients using the REA with a scale of 0 – 5. Comedogenicity was determined using the visual inspection method or the whole mount method. The researchers found that most of the cosmetic products and cosmetic ingredients tested, including products aimed toward acne-prone skin, were comedogenic. They suggested to avoid makeup products that included D&C red dyes, such as makeup blushes, and to use makeup products that are pressed with mineral oil, which is non-comedogenic, instead of with isopropyl myristate. The table below shows the comedogenicity scores for all tested products and ingredients.12
Study #8


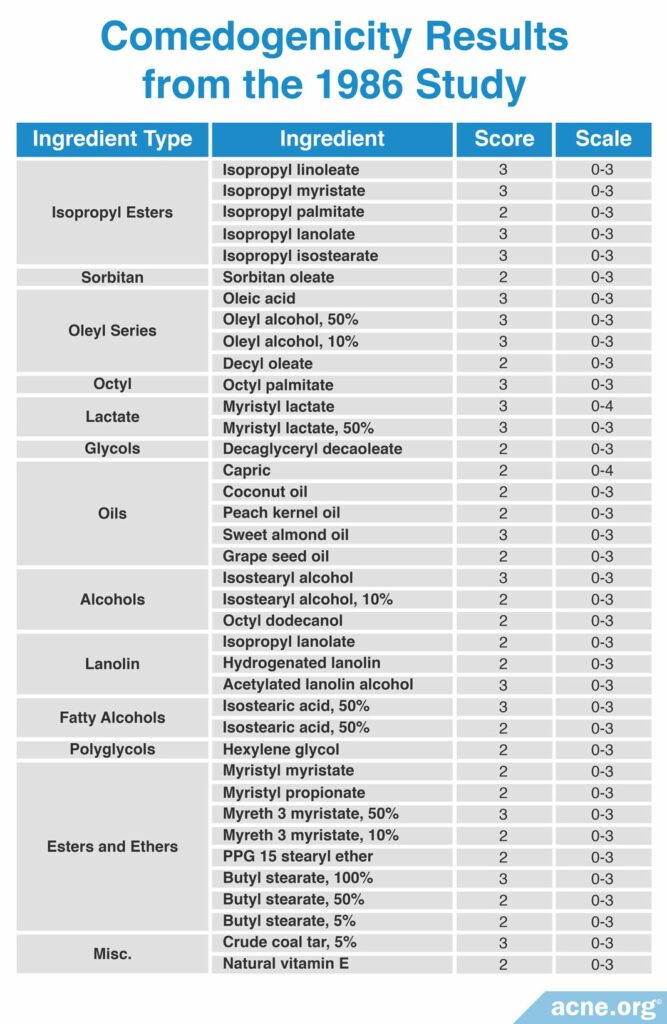
A 1986 study published in Cosmetics and Toiletries reviewed the comedogenicity data from several cosmetic manufacturers and compared their findings. The studies they reviewed used different scoring scales and methods of determining comedogenicity. The researchers found that diluting a comedogenic ingredient could make it less comedogenic. For example, 100% octyl palmitate imparted a comedogenicity score of 3, while 50% octyl palmitate had a comedogenicity score of 1. Therefore, some comedogenic ingredients may become non-comedogenic at lower concentrations. The most comedogenic ingredients are listed in the table below.13
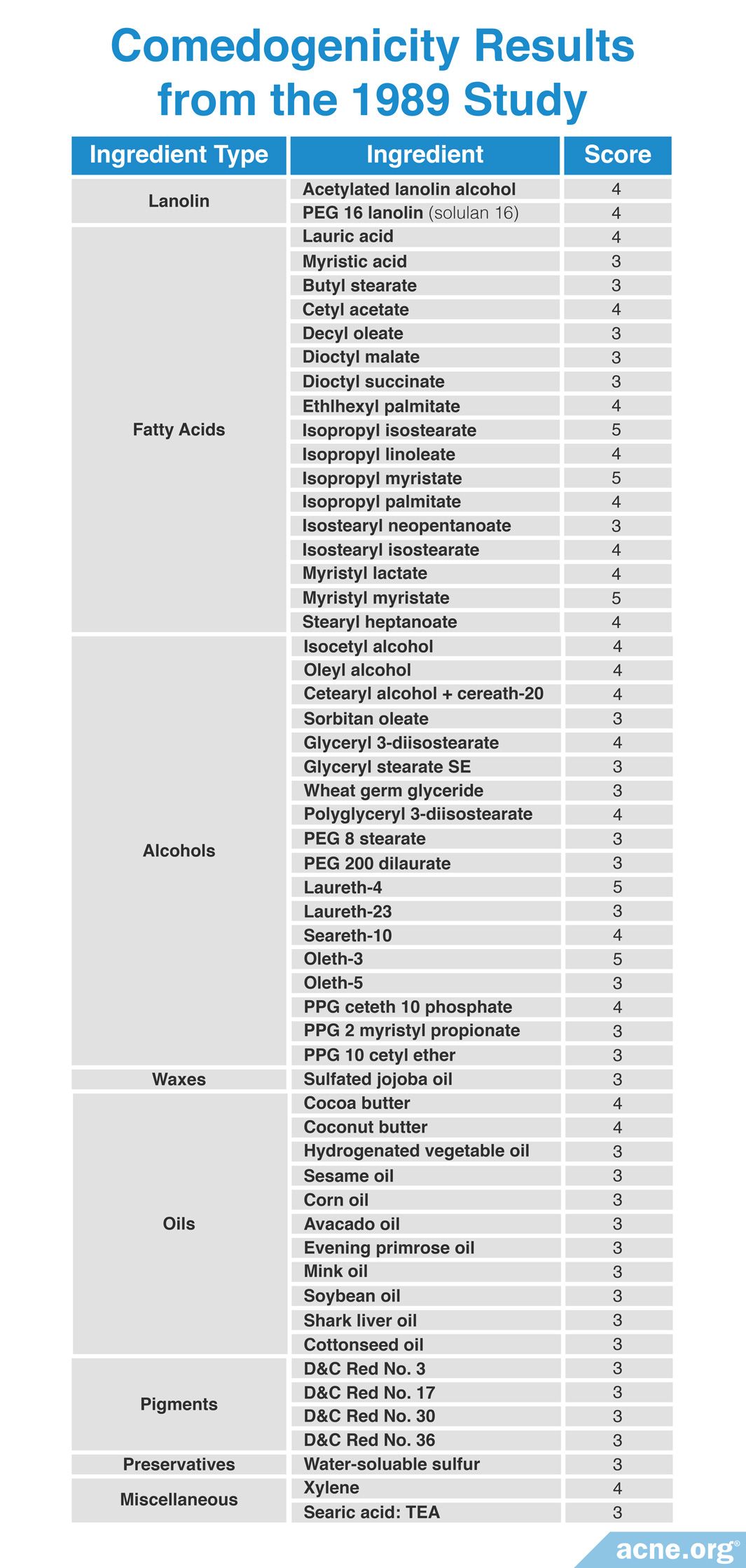
Study #9
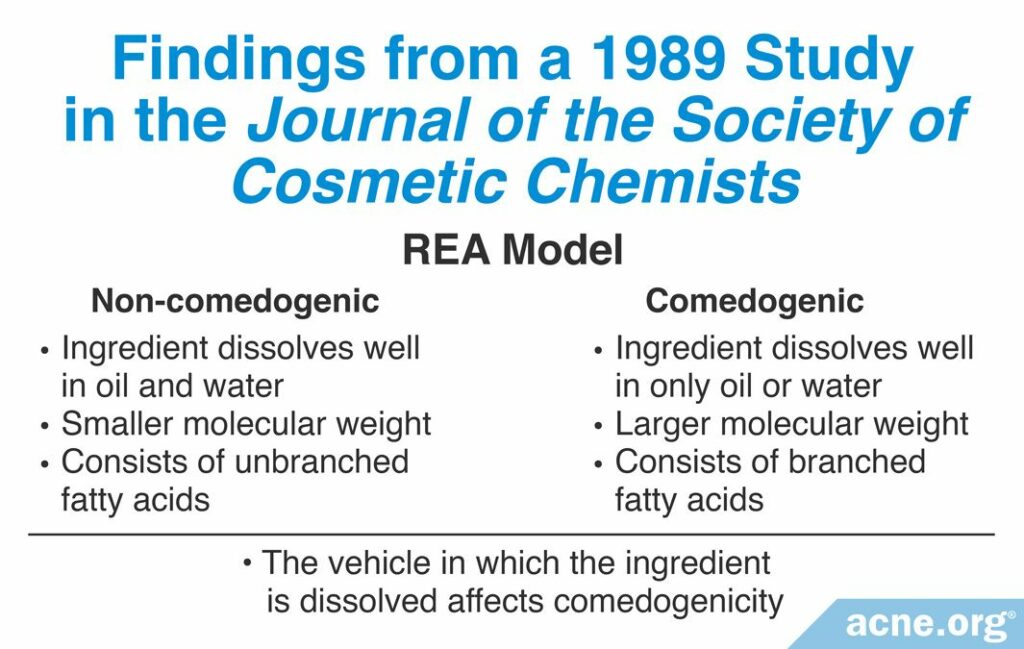

A 1989 study published in the Journal of the Society of Cosmetic Chemists examined more than 200 cosmetic ingredients using the REA assay with a scale of 0 – 5. Although they studied too many ingredients to list here, the researchers found that by comparing so many ingredients they could identify what characteristics made an ingredient more comedogenic than other ingredients. These characteristics include:
1. Ability to penetrate the hair follicle: If an ingredient can dissolve well in oil and in water, then it is more likely to be less comedogenic. If the ingredient dissolves well in only oil or water, then it is more likely to be more comedogenic.
2. Size of the molecule: Ingredients with a smaller molecular weight of 200 – 300 grams per mole are less comedogenic than ingredients with larger molecular weights.
3. Shape of molecule: Fatty acids are molecules with long chains of carbon and hydrogen atoms. In some fatty acid molecules, these chains are straight (unbranched). In some others, they can branch off the main chain in a variety of directions (branched). Branched fatty acids are more comedogenic than fatty acids with unbranched, straight chains.
4. Vehicle in which the ingredient is dissolved: The substance in which the ingredient is dissolved is called a vehicle. Different vehicles can result in different comedogenicity scores for the same ingredient. For example, certain fatty acids have a comedogenicity score of 2 when dissolved in sunflower oil, compared to a score of 0 when dissolved in acetone. Therefore, it is crucial to test the comedogenicity of the ingredient in the final product to determine its true comedogenicity.
Scientists can use these four comedogenicity-determining characteristics to predict whether most ingredients will be comedogenic or non-comedogenic. However, the comedogenicity of oils cannot be predicted using them. This is because all oils are unable to dissolve in water and have molecular weights far greater than 300 g/mol. With these characteristics all oils should be highly comedogenic. However, this is not the case as several oils are non-comedogenic.
Although this study researched too many ingredients to include the full comedogenicity results here, the researchers drew several conclusions about the comedogenicity of multiple classes of ingredient. These include:
- Alcohol-based ingredients that are large or branched tended to show higher comedogenicity. Examples of this group of ingredients include isocetyl alcohol and oleyl alcohol.
- Fatty acids that are large and branched tended to be more comedogenic. Examples include lauric acid and myristic acid.
- Thickening agents, which include silicates, cellulosic polymers, and carbomers, tended to be non-comedogenic.
- Mineral materials generally were non-comedogenic. They include clays, bentonite, kaolin, talc, iron oxide, chromium hydroxide, and titanium dioxide.
- Oils tended to have variable comedogenicity.
Most comedogenic oils included cocoa butter and coconut oil.
Moderately comedogenic oils included sesame oil, corn oil, avocado oil, evening primrose oil, mink oil, soybean oil, and cotton seed oil.
Least comedogenic oils included olive oil, sandalwood seed oil, almond oil, apricot kernel oil, and mineral oil.
Non-comedogenic oils included safflower oil, sunflower oil, and mineral oil.
Mineral oils comprise a class of oils that have been reported with a range of comedogenicity from 0 – 2. However, it is likely that the modern, pure version of mineral oil is non-comedogenic.
- D&C red pigments have a variable degree of comedogenicity: it tends to depend on the vehicle in which it is dissolved. For example, D&C red pigments are not comedogenic when dissolved in volatile propylene glycol, but are comedogenic when dissolved in mineral oil.
- Vitamins E and A have variable degrees of comedogenicity.14
Although all 200 ingredients tested cannot be listed here, the table below lists the most comedogenic ingredients they identified, as measured by having a score of at least 3 on a scale of 0 – 5. Comedogenicity was determined using the histological examination method.
Study #10


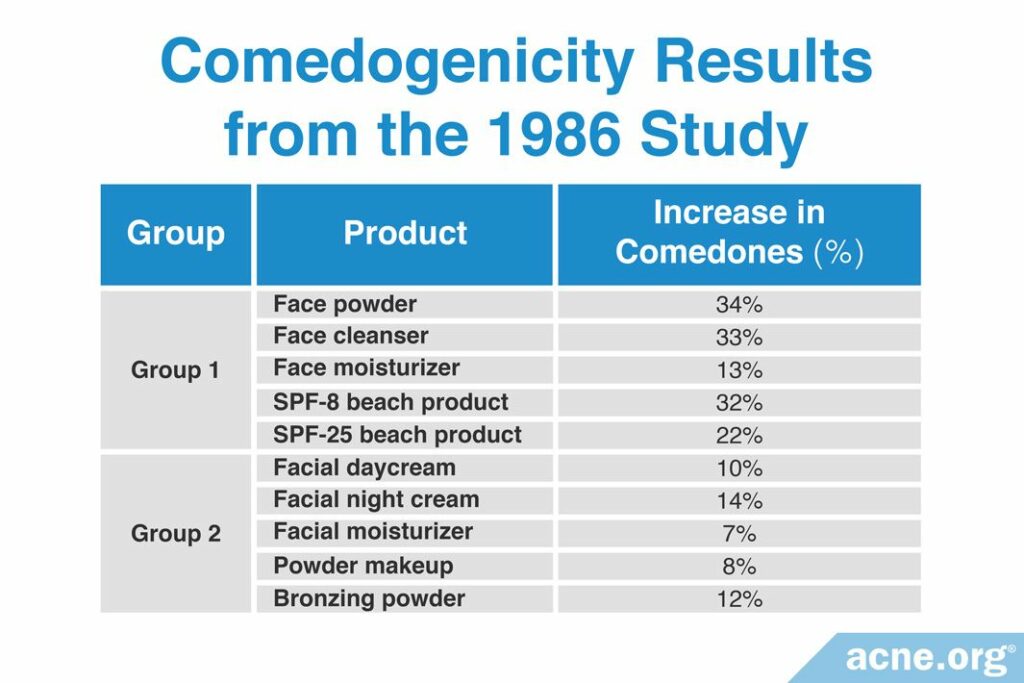
A 2006 study published in the Journal of the American Academy of Dermatology used the human method to determine the comedogenicity of several cosmetic products. To perform this study, they examined the comedogenicity of two groups of cosmetic products.
1. Group 1 tested face powder, facial cleanser, facial moisturizer, and SPF 8 and SPF 25 sunscreens.
2. Group 2 tested facial day cream, facial night cream, facial moisturizer, powder makeup, and bronzing powder.
Instead of measuring with the typical scale used by the other studies, the researchers reported their results as the percentage increase in the number of comedones. In other words, they measured the number of comedones before and after the product was applied, and calculated how much the product increased the number of comedones. The researchers determined that if a product increased the number of comedones by less than 50%, then it was non-comedogenic. They found that all the products they tested were non-comedogenic (see table below).
After they determined that the cosmetic products were non-comedogenic, they examined the ingredient lists of these products and compared their list of comedogenic ingredients to known REA comedogenicity scores. The researchers did this to determine whether the ingredients in the tested products were suspected to be comedogenic or non-comedogenic. Interestingly, their non-comedogenic products contained at least two comedogenic ingredients, as determined by the REA. The researchers answered the question of how they then tested non-comedogenic in two ways.
1. First, they concluded that the REA was more sensitive than the human method, so a comedogenic ingredient as measured by the REA may not be comedogenic in humans.
2. Secondly, they concluded that the comedogenicity score of a cosmetic product depends on several ingredients and the vehicle: a small number of comedogenic ingredients in a cosmetic product that contains many ingredients necessarily does not cause the entire product to be comedogenic.
However, these two conclusions are speculations, and scientists need to perform more research before conclusions can be made. Further, because the researchers were unscrupulous with the label – labeling products as non-comedogenic even if they increased the number of comedones by 40 – 49% – it is possible that a more stringent comedogenicity measurement would have produced slightly different conclusions.15
Study #11
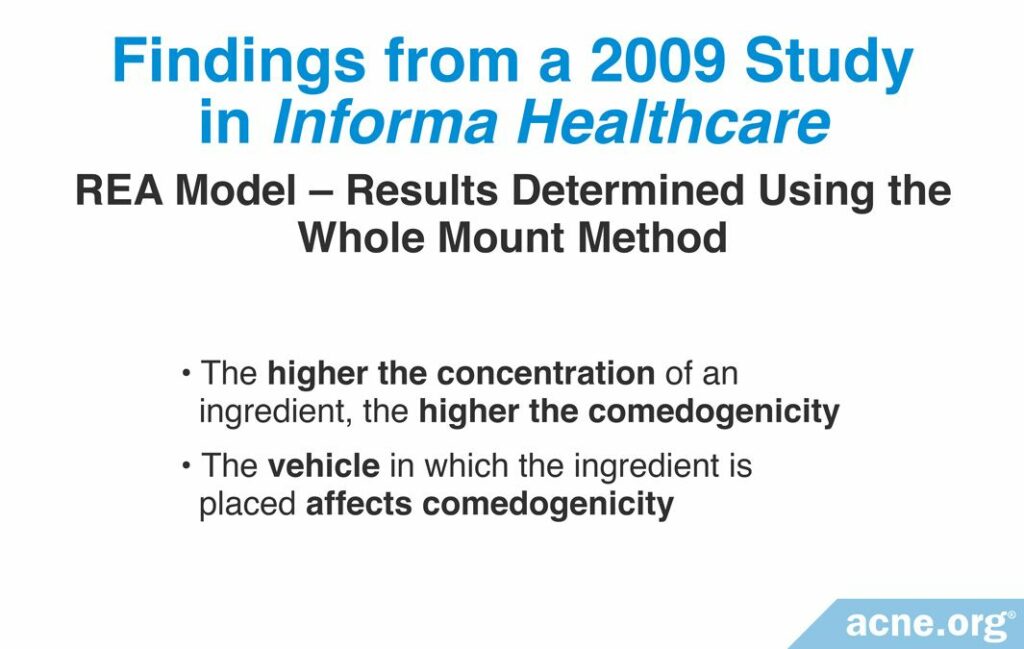

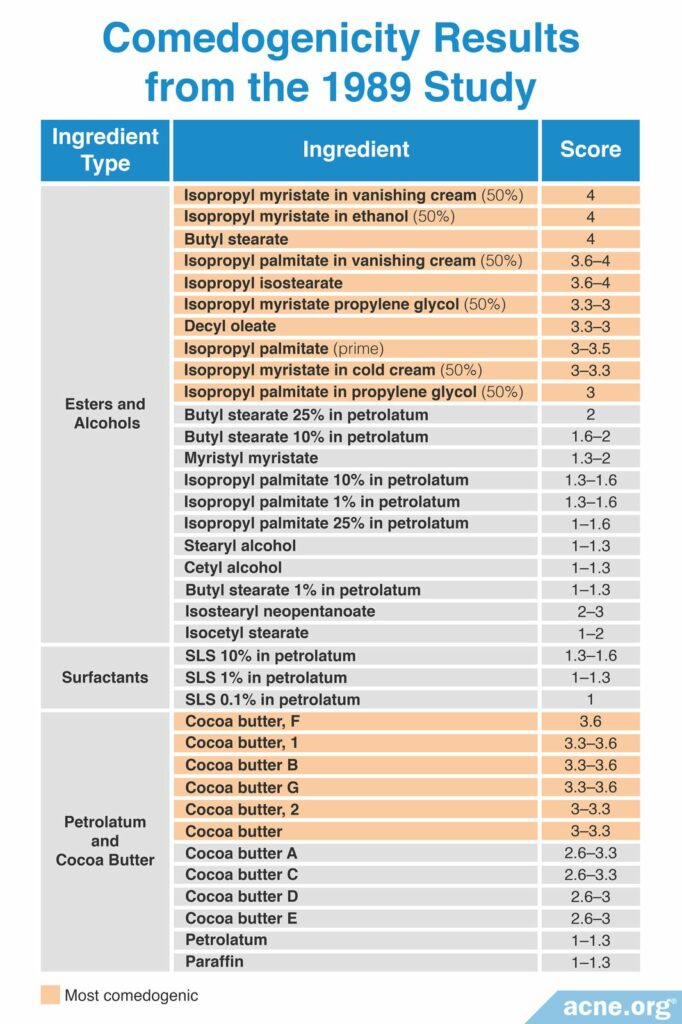
A 2009 study published in Informa Healthcare examined whether the concentration of an ingredient and the vehicle used impacts comedogenicity. To perform this study, the researchers used the REA with a scale of 0 – 4 to examine the comedogenicity of several concentrations of a few cosmetic ingredients, and comedogenicity was determined using the whole mount method. This research found that a higher concentration of the ingredient resulted in higher comedogenicity. In other words, the more ingredient that is used, the more it clogs pores. Additionally, the study found that the type of vehicle in which the ingredient is placed also can affect its comedogenicity. The table below shows the comedogenicity results from this study.16
Choosing a Non-comedogenic Product for Acne-prone Skin
Although we have seen that scientists have performed several studies investigating which ingredients are comedogenic, it can be difficult to analyze the data. This is because each study employs its own method, scale, ingredients, concentrations, vehicle, and duration of testing for each of the ingredients. Also, REA comedogenicity does not apply to humans, necessarily.
Further compounding the confusion, comedogenicity tests are normally performed by applying ingredients at 10% strength. However, in final products, a comedogenic ingredient may be in the formula at a much lower percentage, reducing its chance of clogging pores. Next, since various ingredients interact with one another in product formulas, some final products that contain a comedogenic ingredient may be pore-clogging, while another may not be. As we can see, this is not an exact science.
Still, the data we have is good enough for us to compile two lists that we can use with a fair amount of confidence.
List 1: Avoid: These are ingredients that were found to be comedogenic in two (2) or more REAs or in at least one (1) human study
List 2: Consider Avoiding: These are ingredients that were found to be comedogenic in only one (1) REA
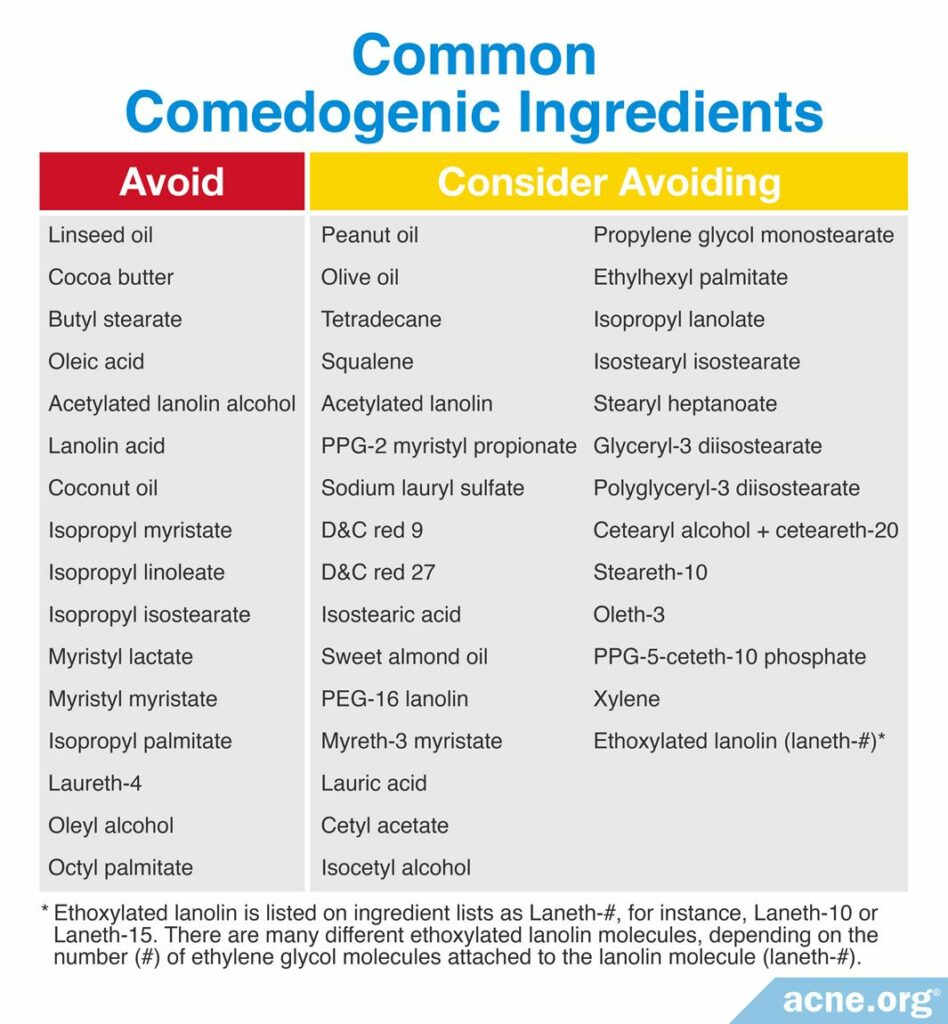
Manufacturers are required to list ingredients in the order of their concentration: the highest percentage ingredient listed first, the second highest listed second, and so on. However, once the concentration dips below 1%, it’s a free-for-all. Manufacturers can list ingredients less than 1% in whichever order they choose. For our purposes, it is prudent to avoid a product if a comedogenic ingredient is listed in the top seven (7) ingredients.

Note on petroleum jelly: According to our rules, Vaseline® petroleum jelly would be on the list of ingredients to avoid. However, The results of scientific studies related to the comedogenicity of Vaseline® petroleum jelly are contradictory. Namely, in several studies using REA and the human method, it was shown to be comedogenic. However, a more recent study disproved this by pointing out that the REA method used previously was not accurate. When repeatedly tested with an improved REA method, and tested on the face of acne patients as well, investigators showed that Vaseline® petroleum jelly does not possess a pore-clogging potential. Thus, it is not included in this list.17

References
- Kligman, A. M. & Katz, A. G. Pathogenesis of acne vulgaris comedogenic properties of human sebum in the external car canal of the rabbit. Arch Dermatol 98, 53 – 57 (1968). https://www.ncbi.nlm.nih.gov/pubmed/4232034
- Kligman, A. M. & Kwong, T. An improved rabbit ear model for assessing comedogenic substances, Br J Dermatol 100, 699 – 702 (1979). https://www.ncbi.nlm.nih.gov/pubmed/157151
- Katoulis, A. C., Kakepis, E. M., Kintziou, H., Kakepis, M. E. & Stavrianeas, N. G. Comedogenicity of cosmetics: a review. J Eur Acad Dermatol Venereol 7, 115 – 119 (1996). https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1468-3083.1996.tb00606.x
- Strauss, J. S. & Jackson, E. M. American Academy of Dermatology Invitational Symposium on Comedogenicity. J Am Acad Dermatol 20, 272 – 277 (1989). https://www.ncbi.nlm.nih.gov/pubmed/2521642
- Tucker, S. B., Flannigan, S. A., Dunbar, M. & Drotman, R. B. Development of an Objective Comedogenicity Assay. Arch Dermatol 122, 660 – 665 (1986). https://jamanetwork.com/journals/jamadermatology/article-abstract/547251
- Groh, D. G., Mills, O. H. & Kligman, A. M. Quantitative assessment of cyanoacrylate follicular biopsies by image analysis. J Soc Cosmet Chem 43, 101 – 112 (1992). http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.469.7764&rep=rep1&type=pdf
- Kligman, A. M. & Mills, O. H. Acne cosmetica. Arch Dermatol 106, 843 – 850 (1972). https://www.ncbi.nlm.nih.gov/pubmed/4264346
- Mills, A. M. & Kligman, O. H. Acne detergicans. Arch Dermatol 111, 65 – 68 (1975). https://jamanetwork.com/journals/jamadermatology/article-abstract/534711
- Mills, O. H. & Kligman, A. M. A human model for assessing comedogenic substances. Arch Dermatol 118, 903 – 905 (1982). https://www.ncbi.nlm.nih.gov/pubmed/7138047
- Mills, A. M. & Kligman, O. H. Comedogenicity of sunscreens. Arch Dermatol 118, 417 – 419 (1982). https://jamanetwork.com/journals/jamadermatology/article-abstract/543247
- Morris, W. E. & Kwan, S. C. Use of the rabbit ear model in evaluating the comedogenic potential of cosmetic ingredients. J Soc Cosmet Chem 34, 215 – 225 (1983). https://pdfs.semanticscholar.org/5be8/c6c2a7bb0509ac8bf0e5ea661adcd859bd6f.pdf
- Fulton, J. E., Pay, S. R. & Fulton, J. E. Comedogenicity of current therapeutic products, cosmetics, and ingredients in the rabbit ear. J Am Acad Dermatol 10, 69 – 105 (1984). https://www.ncbi.nlm.nih.gov/pubmed/6229554
- Lanzet, M. Comedogenic effects of cosmetic raw materials. Cosmet Toilet 101, 63 – 72 (1986).
- Fulton, J. E. Comedogenicity and irritancy of commonly used ingredients in skin care products. J Soc Cosmet Chem 43, 321 – 333 (1989). https://pdfs.semanticscholar.org/578c/d23064f4be5f9f623e9cb9dbfe4a6c29eef2.pdf?_ga=2.82427924.366179922.1580174937-240515812.1580174937
- Draelos, Z. D. & DiNardo, J. C. A re-evaluation of the comedogenicity concept. J Am Acad Dermatol 54, 507 – 512 (2006). https://www.ncbi.nlm.nih.gov/pubmed/16488305
- Nguyen, S. H., Dang, T. P. & Maibach, H. I. Handbook of Cosmetic Science and Technology, 3rd Edition. Informa Healthcare 583 – 586 (2009). https://www.academia.edu/30003534/Cosmetic_Science_and_Technology_Third_Edition
- Kligman, A. M. Petrolatum is not comedogenic in rabbits or humans: A critical reappraisal of the rabbit ear assay and the concept of “acne cosmetic.” J Soc Cosmet Chem 47, 41 – 48 (1996).
 Acne.org Products
Acne.org Products