Mild Liquid Facial Cleansers Are Best for Acne-prone Skin. Avoid Soap.

The Essential Info
The name of the game when it comes to washing acne-prone skin is to stay as gentle as possible and not irritate the skin. Facial cleansers are much milder and less irritating than soap. So the answer is clear, opt for a facial cleanser and completely avoid soap.
How do you find an ideal facial cleanser? Follow these tips:
- Liquid varieties are preferable to bars.
- Products with a pH between 4 and 6 are best for acne-prone skin. You can find a cleanser close to this pH range by choosing one that is specifically made for the face and states on the label that it is “ultra-gentle” or “made for sensitive skin.”
When you are cleansing, make sure to rinse completely. Even a mild cleanser can cause skin irritation if left on the skin.

The Science
- How Soaps and Cleansers Work
- What’s the Difference Between Soaps and Cleansers?
- The Ingredients in Soaps and Cleansers
- How Soaps and Cleansers Affect Acne-prone Skin
- Choosing the Right Cleanser
Washing the skin is important because it removes dirt, sweat, and excess skin oil and allows acne medications to penetrate into the skin.
However, some soaps and cleansers can irritate your skin, and when it comes to caring for acne-prone skin, reducing irritation is crucial.
All soaps are irritating, so it is best to completely avoid using soap on any area of acne-prone skin.
When it comes to cleansers, the ideal cleanser for acne-prone skin needs to be gentle, meaning that it needs to be:

- Non-irritating: It should not over-dry the skin or be harsh to it.
- Non-comedogenic: It should not clog skin pores.
- Non-allergenic: It should not trigger an allergic reaction, such as redness, swelling, itching, or a rash.1
To understand why all soaps and some cleansers irritate the skin more than others, we need to take a quick look at how soaps and cleansers work.
Caution – Deep Science Ahead: We are about to take a deep dive and understand all there is to know about soaps vs. cleansers, how they work and affect the skin, the various types of each, and how to choose the right one.
If you’re simply interested in what to use when washing your acne-prone skin, it is fairly simple: Never use soap. Instead, use an ultra-gentle liquid cleanser that is specifically made for the face.
How Soaps and Cleansers Work
If you have ever tried to make your own salad dressing, you probably know that oil and water do not mix. When you wash your skin with just warm water, without using a soap or cleanser, the water does not sufficiently mix with any grease or oil that may be on your skin. As a result, too much oil or grease may still remain on the skin surface.
This is why you need the help of a cleanser to remove oil or grease from the skin. Soaps and cleansers contain ingredients called surfactants, which is short for “surface active substances.” Surfactants act as go-betweens, allowing for oil and water to mix, and facilitating the removal of oil and dirt from the skin during washing.
How soaps and cleansers work: The full scoop
Chemists classify all kinds of substances based on whether they can mix with water. If a substance mixes well with water, it’s called hydrophilic, which means “water-loving.” Table salt is an example of a hydrophilic substance: if you pour some salt into a pot of water, the two will mix easily.
Conversely, if a substance does not mix well with water, it is called hydrophobic, which means “water-fearing.” Oil, grease, and fat are all hydrophobic. These hydrophobic substances mix easily with each other, but not with water.
Soaps and cleansers have a difficult task. They have to be able to mix with things like grease in order to “grab onto” them and remove them from the skin. On the other hand, soaps and cleansers need to be able to mix with water so that you can rinse them off afterwards. This is why they contain two parts.
1. A hydrophilic “head” that can mix with water
2. A hydrophobic “tail” that can mix with oil and grease
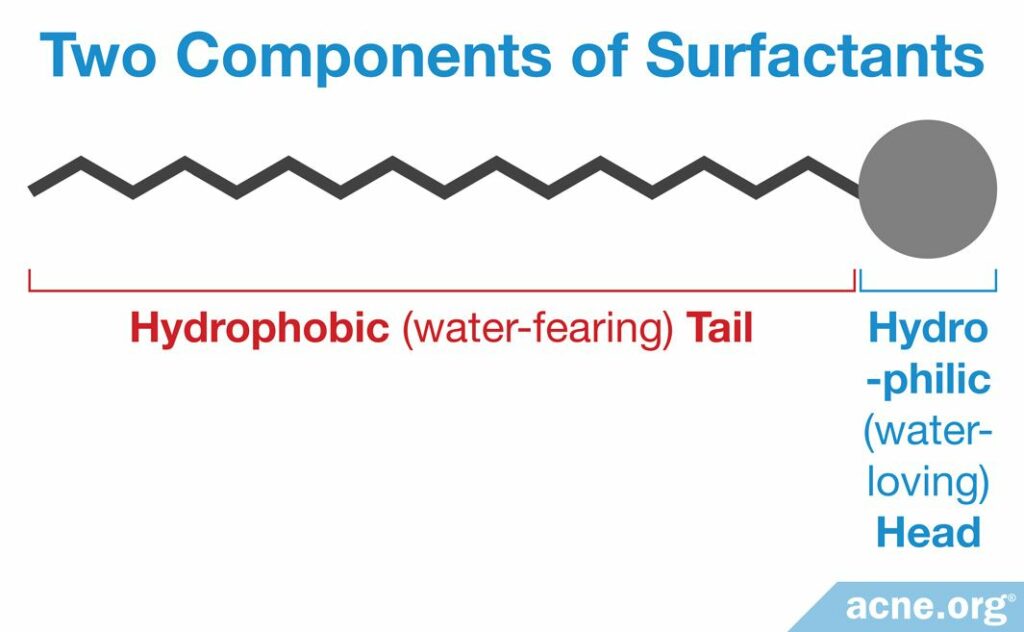
Of course, these heads and tails exist at the molecular level, so you will not be able to see them by looking at your soap or cleanser.
When you wash oily skin with water and soap or a cleanser, the hydrophobic tails in the soap or cleanser surround the droplets of oil, while the hydrophilic heads mix with the surrounding water.
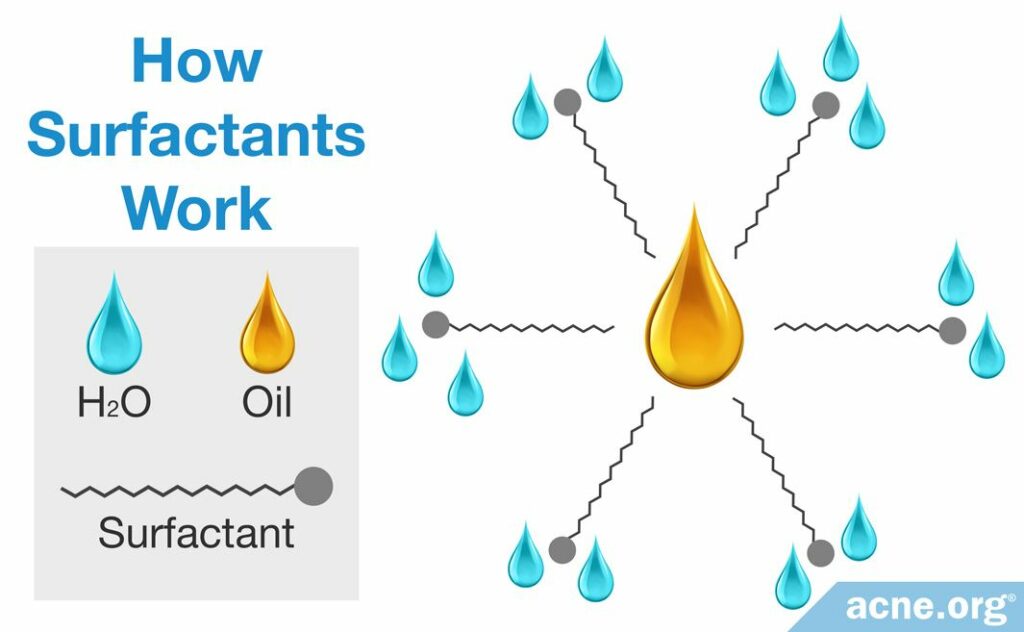
In this way, soaps and cleansers assist in washing by acting as middlemen.
What’s the Difference Between Soaps and Cleansers?
Now that we know how soaps and cleansers work, let’s examine the differences between them in order to understand why cleansers are better for acne-prone skin.
- Soap — the oldest known type of surfactant: The word “soap” refers to a specific class of substances made by a process called saponification, which involves cooking a fat or oil together with a chemical like sodium hydroxide.
- Cleanser — technically known as syndet — a newer type of surfactant: The word “syndet” is short for “synthetic detergent.” There are many different types of syndets, and therefore many different methods of making them.
Both soaps and syndets can come in either bar or liquid form.
The Ingredients in Soaps and Cleansers
Ingredients in soaps
People have been producing soap for thousands of years, and it remains popular today. However, not everything that we call “soap” in everyday language is actually soap in the chemical sense. True soap is made by a process, called saponification, involving two components.
- A fat or oil
- A base (a type of chemical that is not acidic, such as sodium hydroxide)2
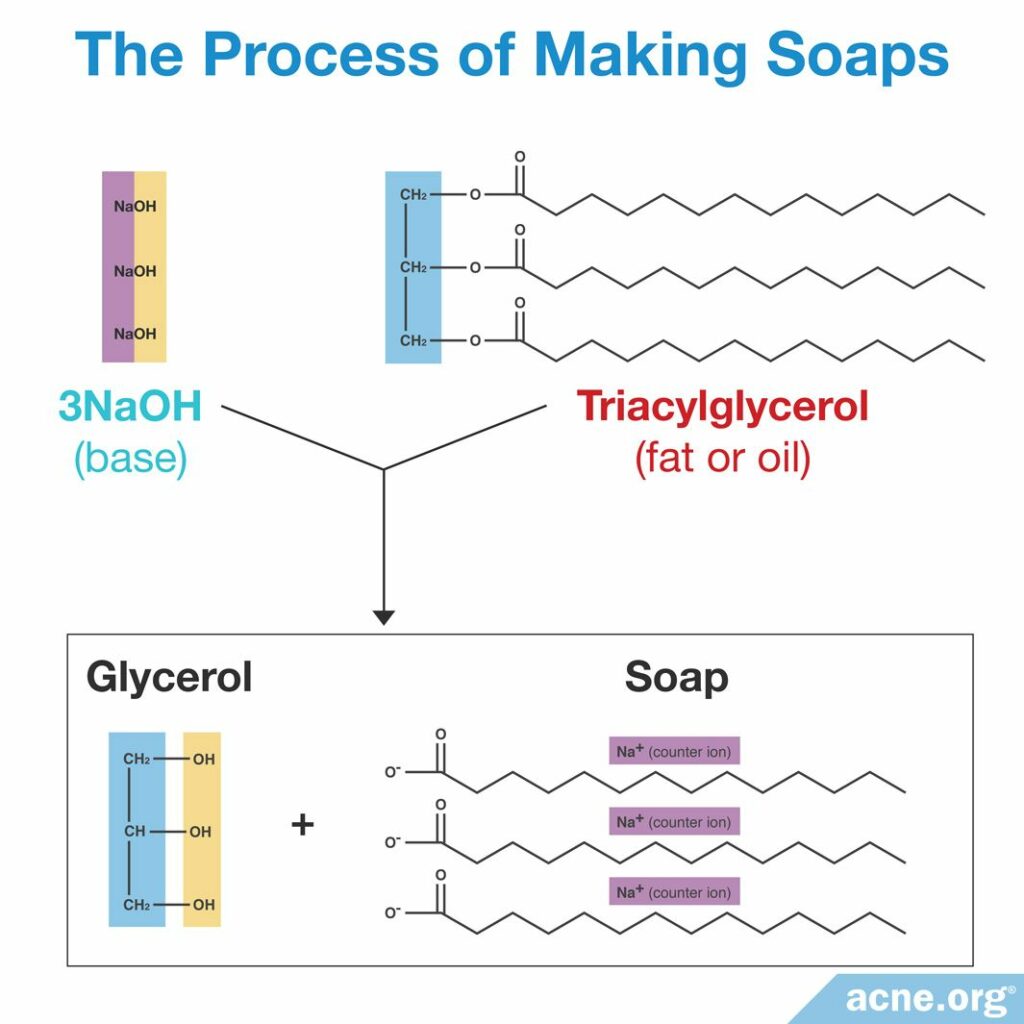
You can usually tell which fat or oil and which base were used in making a particular soap by looking at the names of the ingredients. The second part of the chemical name usually comes from the fat or oil, and the first part, from the base.
The table below shows common soap ingredients. The component in red comes from the fat or oil, and the component in blue comes from the base.
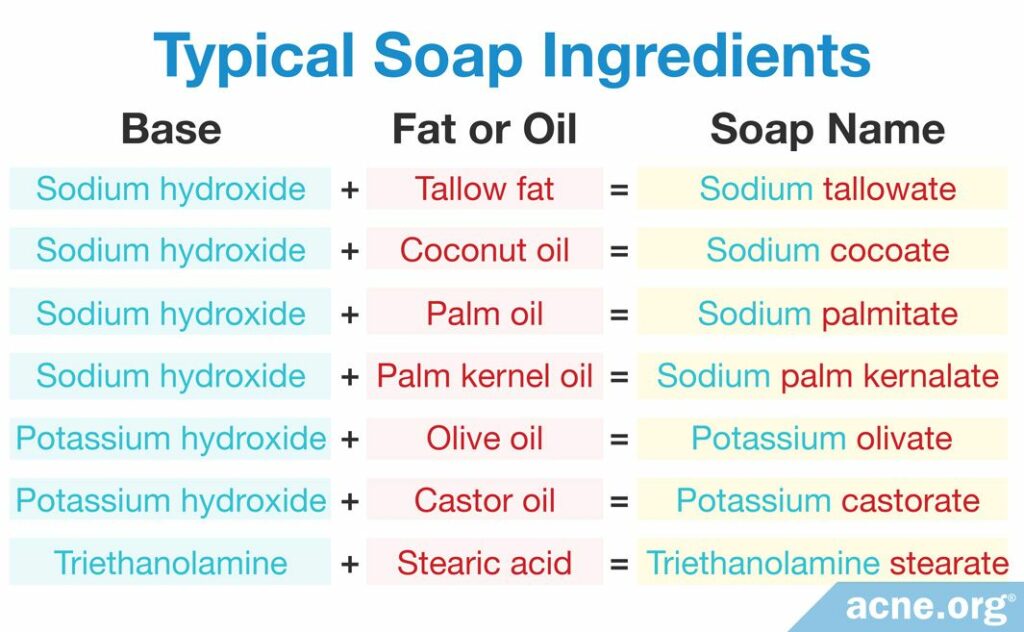
If you are not sure whether a product is an actual soap, check the list of ingredients on the back of the product. If any of the ingredients appear in the table above, avoid using the product, and opt for a cleanser instead.
Ingredients in soaps: The full scoop
The fat or oil for making soap can be an animal fat, like tallow or lard, or a vegetable oil, like coconut oil or olive oil. Bar soap today usually contains a mixture of both animal fat and vegetable oil, with the most common combination being 75 – 85% tallow and 15 – 25% coconut oil. However, purely vegetable-based soaps are also popular. In such soaps, the oil component is usually 75 – 85% palm oil and 15 – 25% coconut oil.
The base for making soap is usually sodium hydroxide, potassium hydroxide, or an ammonium compound like triethanolamine.
The qualities of the soap depend on which fat or oil and which base are used. In other words, the specific ingredients determine how harsh a given soap will be on the skin. For example, a soap made from sodium hydroxide is harsher than one made from potassium hydroxide.
Ingredients in cleansers
What we call “cleansers” in daily life are technically known as syndets (synthetic detergents). Manufacturers have been making syndets since the late 1940s, and there is no single method of producing them.
Similar to soaps, syndets contain surfactants as the main ingredients, but syndet surfactants are different from soap surfactants. There are four types of surfactants found in syndets.
- Anionic (negatively charged)
- Cationic (positively charged)
- Amphoteric (containing both positive and negative charges)
- Nonionic (not charged)2,3
A cleanser can contain several different types of surfactants together. The types of surfactants in a cleanser determine how harsh that cleanser will be on the skin. In general, we can rank surfactants in cleansers as follows, from the harshest to the mildest:
- Anionic
- Amphoteric
- Nonionic
Cationic surfactants are rarely incorporated into cleansers, so we do not have enough data on how irritating they may be to the skin.
Ingredients in cleansers: The full scoop
As we have already seen, surfactants consist of a hydrophilic head and a hydrophobic tail. The biggest difference between different types of surfactants is the kind of hydrophilic head they have, which determines its name. For example, if a surfactant has an anionic (negatively charged) head, it is called an anionic surfactant.
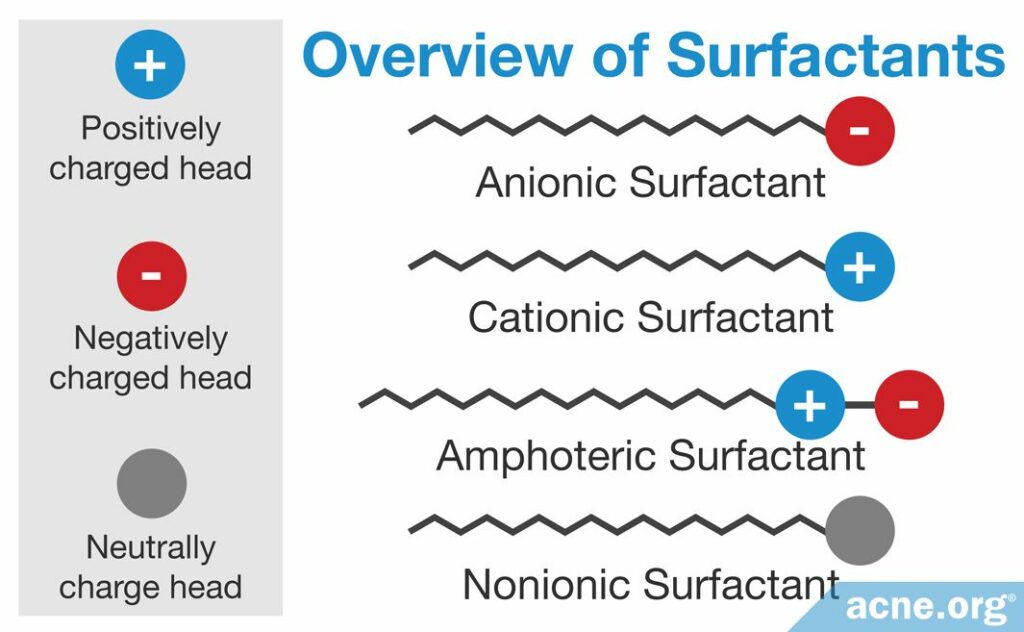
Most facial cleansers consist either of anionic surfactants alone or of a mixture of anionic and amphoteric surfactants.2,3
How Soaps and Cleansers Affect Acne-prone Skin
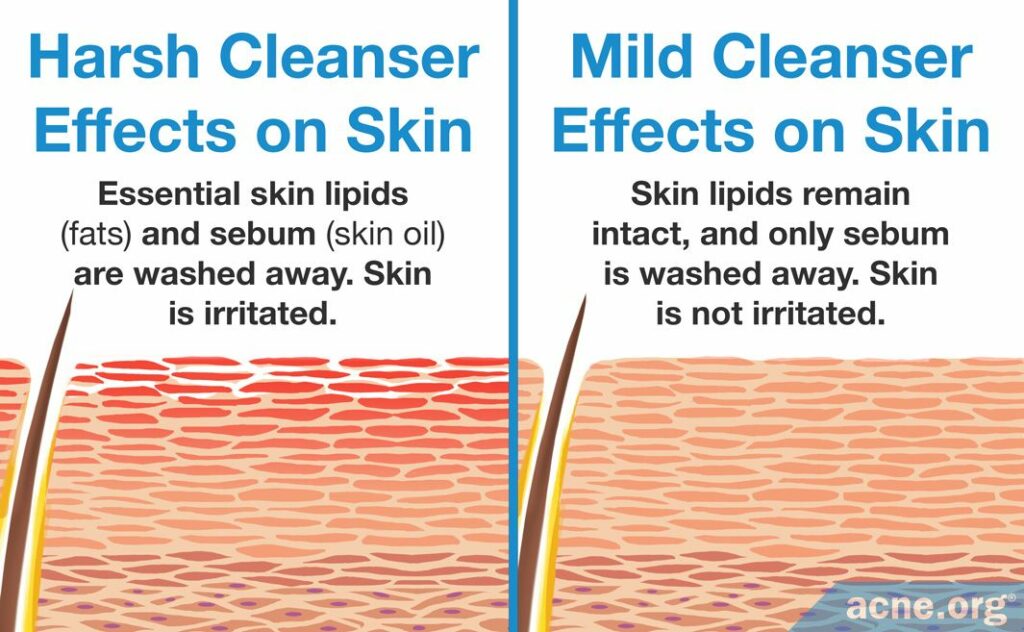
People with acne typically produce too much skin oil, some of which ends up on the skin surface and gives the skin a greasy appearance. To effectively wash off this excess oil and to prepare the skin for acne treatments, either soap or cleanser is required.1
On the other hand, soaps and cleansers can interfere with the skin barrier–in other words, they can damage the skin. This means that while we cannot do away with using a soap or cleanser, we need to select the gentlest one possible to avoid damaging the skin. Since research shows that cleansers tend to be less harsh to the skin than soaps, it is better to use a cleanser, and to choose the mildest one.3-12
The skin barrier
The skin barrier is the outer layer of the skin, also known as the stratum corneum. You can think of the stratum corneum as a protective wall between your body and the outside world. A wall consists of bricks (skin cells) that are held in place by mortar (constitutive lipids).3,4 The skin barrier is like our outer “armor,” protecting our bodies from physical damage, from chemicals in our environment, and from bacteria and viruses. Most importantly, the skin barrier keeps the skin hydrated by preventing it from losing too much water.
A good soap or cleanser needs to perform a difficult balancing act. On the one hand, it needs to remove enough oil from the skin surface so that the skin does not look greasy, and so that acne treatments can penetrate the skin. On the other hand, a good soap or cleanser must not damage the constitutive lipids that are crucial for the skin barrier.4
Potential side effects of soaps and cleansers
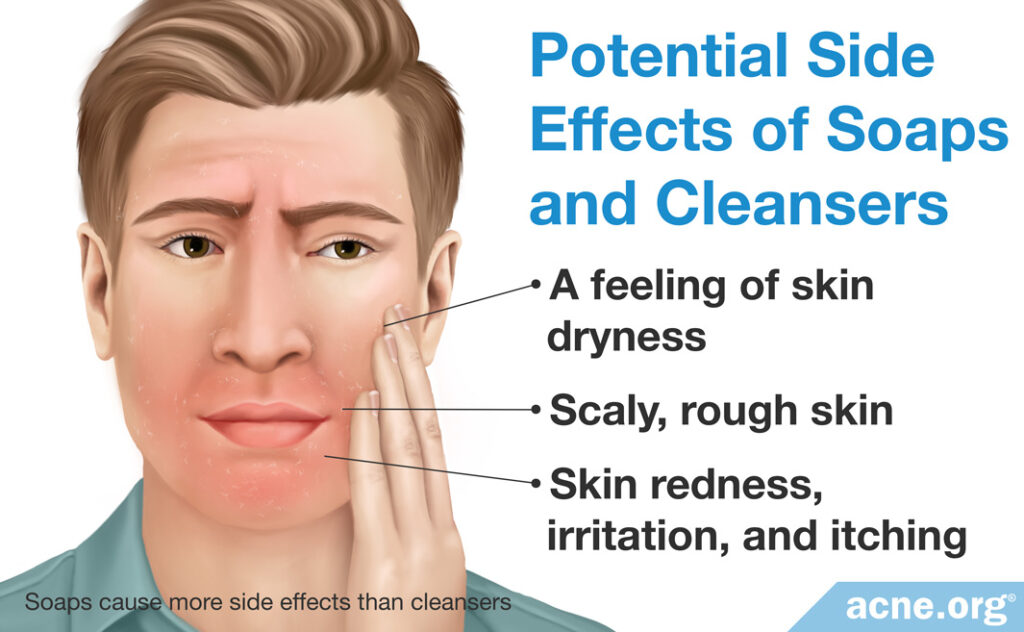
Both soaps and syndets can cause side effects. These can include:
- A feeling of skin dryness
- Scaly, rough skin
- Skin redness, irritation, and itching
Soaps are more likely than syndets to cause side effects. In addition, if you have “hard water” in your home, which would mean it contains many minerals, washing your skin with soap may react with the minerals to create residues that can clog skin pores. As a rule of thumb, acne-prone people should avoid using soap.
The surfactants in modern facial cleansers are usually relatively mild and rarely produce major side effects. Still, people with acne-prone skin should choose the gentlest cleanser possible, and be careful to choose one specifically made for the face. It can also be helpful to look for terms like “mild,” “for sensitive skin,” and “hypoallergenic” on the label.
Potential side effects of soaps and cleansers: The full scoop
More specifically, side effects from soaps and cleansers can include:
- After-washing tightness
After-washing tightness is a sense of skin dryness that occurs 5 – 10 minutes after washing with a hash soap or cleanser. The feeling may be only temporary and does not necessarily mean that the skin barrier is impaired.4
- Skin dryness
Skin dryness can occur after washing with a harsh soap or cleanser. This happens because harsh surfactants can remove some of the constitutive lipids from the skin and thus impair the skin barrier, leading to scaly, rough skin.4
- Skin redness, irritation, and itching
Surfactants found in some harsh soaps and cleansers can penetrate into the stratum corneum and may trigger the body’s immune response, leading to redness, irritation, and itching of the skin.4
When it comes to soaps, those made from sodium hydroxide are more likely to cause irritation than soaps made from potassium hydroxide. You can tell that a soap was made from sodium hydroxide if its name begins with the word “sodium,” for example, “sodium tallowate.” Although acne-prone people should avoid all soaps, soaps made from sodium hydroxide are particularly harmful.
When it comes to cleansers made from synthetic ingredients, those containing anionic surfactants are the most irritating to the skin, followed by amphoteric and nonionic surfactants.4 However, even this can present confusion, since it also depends on the amounts of each used in a cleanser. For instance, even if a cleanser contains a potentially “harsh” surfactant, it may only be included at a very small concentration.
Choosing the Right Cleanser
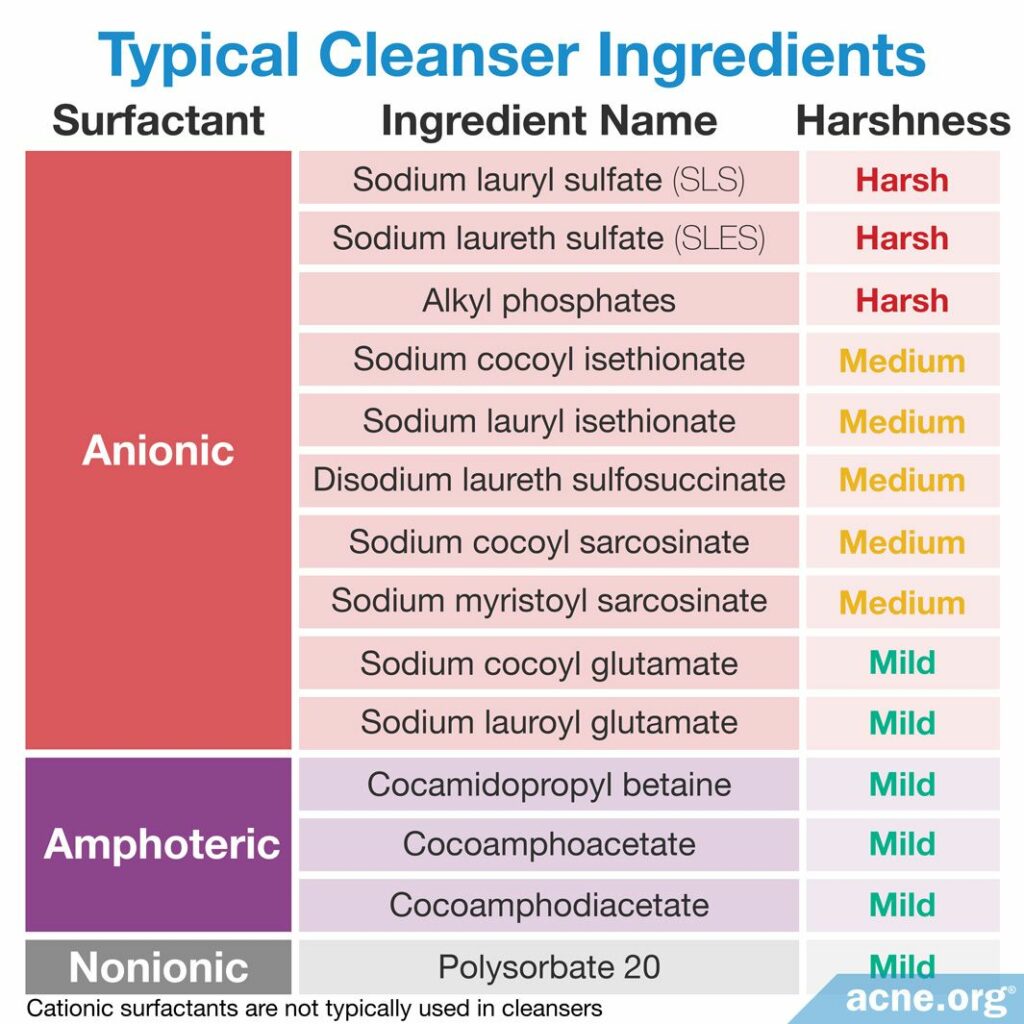
The table below lists some common cleanser ingredients, which we have ranked from harsh to mild. When you buy a cleanser, check the ingredients on the back and do your best to avoid any products that contain ingredients that are labeled as “harsh” in the table. If any of the “medium” surfactants are included in the first seven ingredients in the cleanser, it may also be best to keep shopping for a milder cleanser.
Another approach to testing the harshness of your cleanser
Besides viewing the list of ingredients, there is another way to test the harshness of your cleanser–if you’re feeling extremely motivated. For example, if none of the ingredients listed on the back of your cleanser are in this table, you can still gauge how harsh the cleanser will be by buying something called a pH tester at your drugstore. A pH tester is usually a paper strip that you can dip into the cleanser.
When you dip the paper strip into your cleanser, the strip will change color. Each color corresponds to a particular pH (acidity/basicity level), which you can look up on the color chart that comes with the pH tester. In general, you should use cleansers that possess a pH between 4 and 6, as these match the natural pH of human skin and are thus less likely to cause problems.6-8
Choosing the Right Cleanser: The Full Scoop
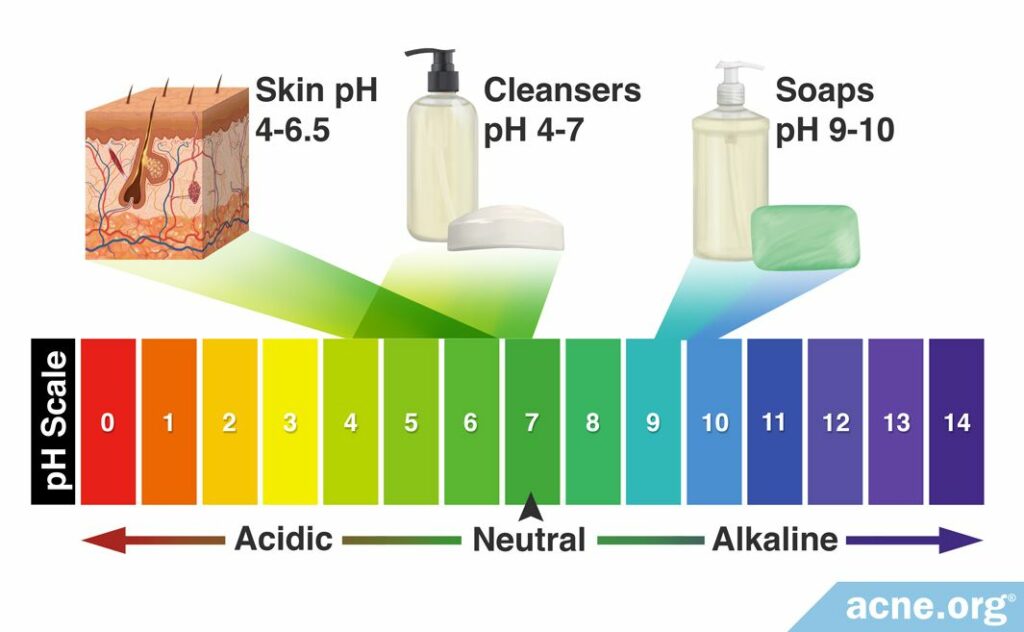
To fully understand why some soaps and cleansers can harm the skin, we need to discuss something called the pH scale.
The pH scale
This is a scale from 0 – 14 that describes how acidic a substance is. The lower the pH, the more acidic a substance is. Conversely, the higher the pH, the more basic/alkaline the substance. In general, acids tend to lend a sour taste, while bases usually have a bitter taste. Substances in the middle of the scale have a pH of 7 and are considered neutral. Pure water is an example of a neutral substance.
Abnormal skin pH can contribute to acne
Healthy skin has a pH of 4 – 6, depending on the body part, which means that the skin is normally mildly acidic. Research shows that when we use soap or a cleanser with a pH that is drastically different from these values, we can damage the health of the skin.6 In particular, changing the pH of the skin can cause more bacteria to grow on it.
Normally, areas of the body that produce an abundance of sebum, such as the face, provide a home for the bacteria C. acnes. While this bacteria lives inside the skin of most people, it can give rise to acne lesions when its growth becomes excessive. The most favorable pH values for C. acnes growth are 6 – 6.5.5
Several studies have tried to compare soaps and cleansers with different pH values.
Studies comparing soaps and cleansers with different pH values

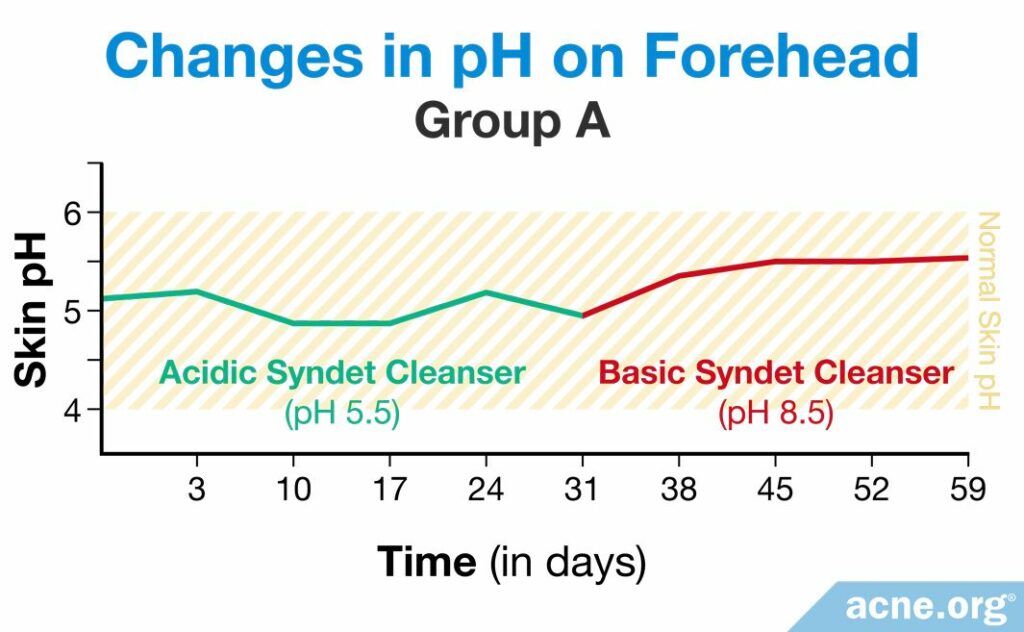
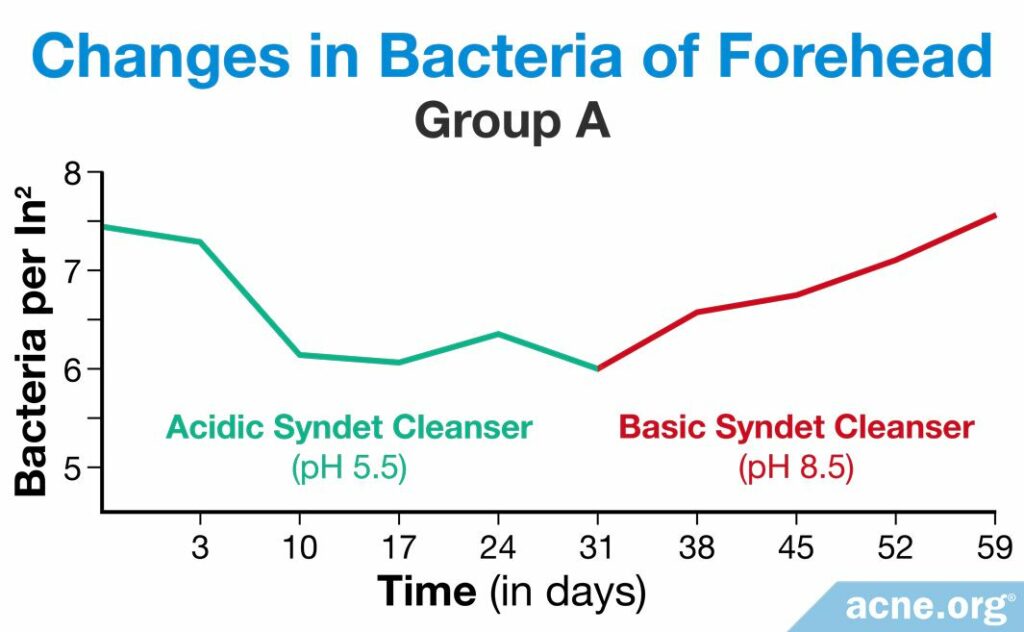
A study published in 1991 in the Journal of the Society of Cosmetic Chemists looked into how people’s skin pH and the amount of bacteria on the skin changed after using two different syndets. One syndet was acidic, with a pH of 5.5, and the other was basic, with a pH of 8.5. Five men and five women between the ages of 24 and 35 participated in the study and were divided into two groups, A and B. Each participant washed his or her face twice a day. This method of testing cleansers is called the “in-use method,” which involves the participants using the product in a natural way. For the first eight weeks, group A used the acidic syndet and group B used the basic syndet, after which point they switched for the next four weeks.6
The researchers assessed skin pH values once per day between the twice-daily cleansing periods, at least six hours after the initial daily cleansing. They took these measurements on days 1 – 3 and on day 10, 17, 24, 31, 38, 45, 52, and 59. In group A, the researchers found that the pH of the skin did not change while the participants were using the acidic syndet, but increased when they began to use the basic one. As the pH increased, so did the amount of C. acnes on the skin.6
In group B, the pH of the skin rose immediately as they began to use the basic syndet, and the amount of C. acnes increased accordingly. When the volunteers in group B began to use the acidic syndet, the pH of the skin decreased, along with the amount of bacteria.6
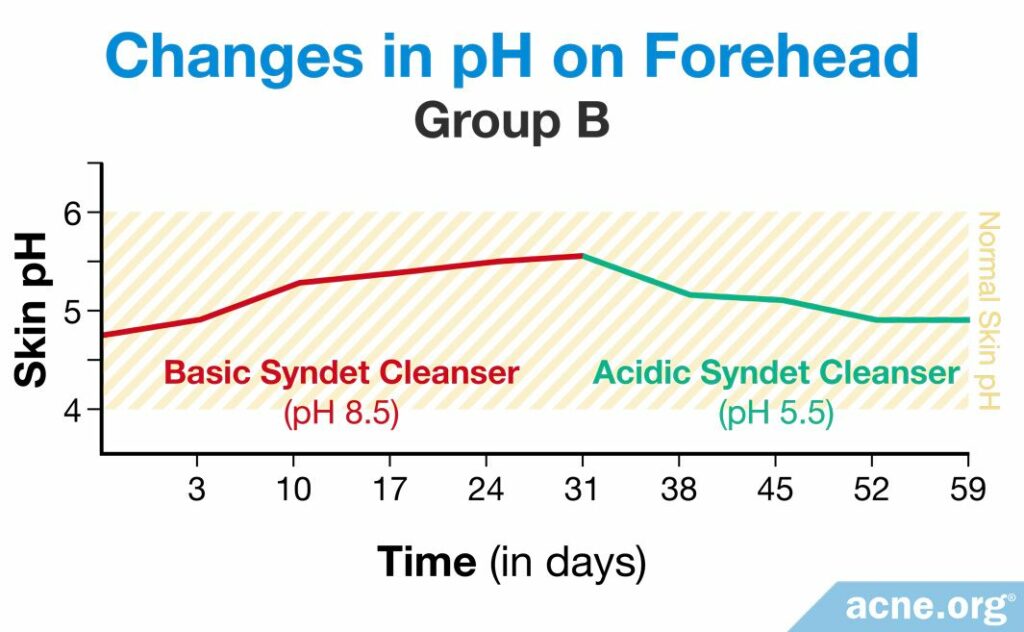
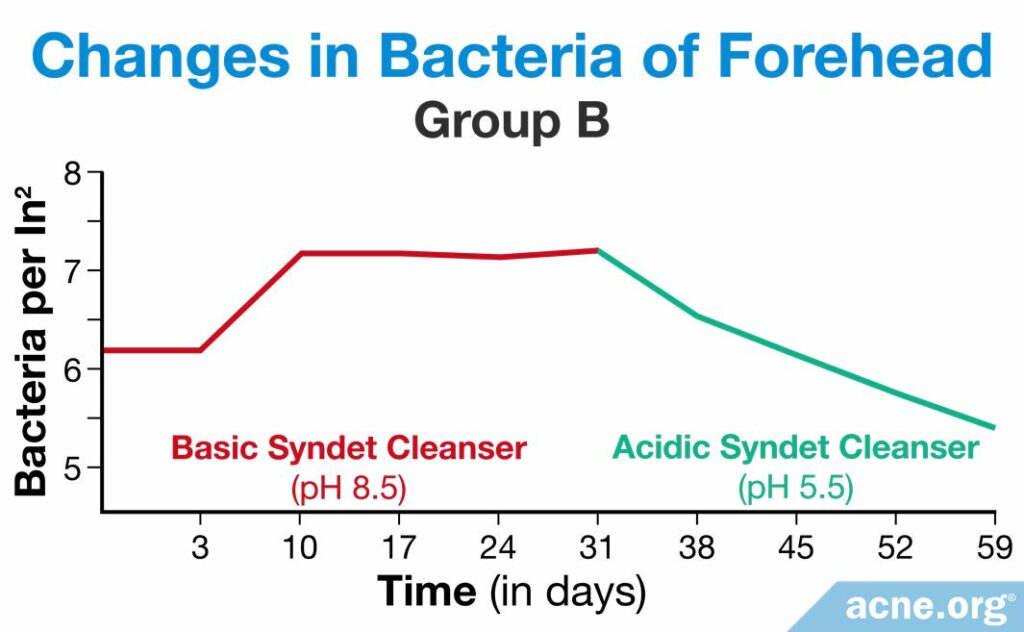
The results of this study show that it is important to use slightly acidic cleansers in order to maintain normal skin pH and to prevent the overgrowth of bacteria which can contribute to acne.

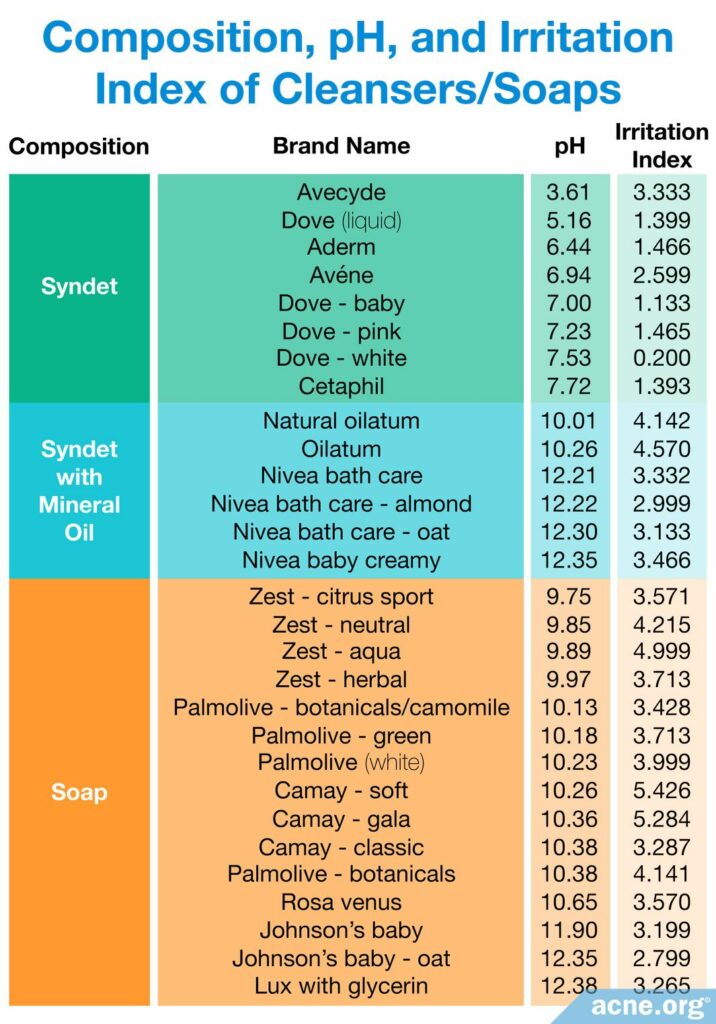
Another study, published in 2002 in the International Journal of Dermatology, compared the irritation effects of 12 common soaps and 17 cleansers marketed for dry skin. The test participants were 30 healthy adults between the ages of 18 and 41, half of whom were female and the other half, male. The researchers conducted a so-called “chamber test,” which meant that the soap or cleanser was placed on absorbent paper and fixed onto the participant’s forearm for 24 hours on the first day and then for several hours every day over another four days. This type of test reveals small differences in side effects between different products, but since the conditions of the test are extreme, the results should be interpreted with caution.7
Based on the reaction of the skin to the product after five days, the researchers assigned each product an irritation index. A higher index means that the product caused greater irritation. Only six of the products earned an irritation index of 1 or less and were considered to be non-irritant. All of these non-irritant products were syndets–in other words, cleansers, not soaps. In terms of pH, all of them were either slightly acidic or close to neutral.7
The remaining soaps and cleansers all caused some degree of irritation. Among the remaining cleansers, some had a pH that was too low, such as 3.6, and some had a pH that was too high, such as 9.8 and above. The soaps all had a pH that was too high–that is to say, they were all too basic.7
The researchers found that products with a pH of 10 were the most irritating. Most of these highly irritant products were soaps, but two cleansers were also in this group.7
The results of this study confirm that people with sensitive skin should avoid soap and choose cleansers with a slightly acidic pH, or at least a pH that is close to neutral.

A third study, published in 2001 in the journal Skin Research and Technology, also compared three products with different pHs: an almost-neutral syndet with a pH of 6.9, a classic soap with a pH of 9.6, and a basic soap with a pH of 10.5. First, a group of 50 female participants between the ages of 18 and 50 tested the syndet and classic soap with the in-use method. This experiment did not reveal any major differences between the two products in terms of side effects on the skin.8
In the second part of the study, 20 female participants between the ages of 18 and 55 tested all three of the products using the chamber method. The chamber method involves much more contact between the syndet or cleanser and the skin than the in-use method. As a result, participants experienced more side effects in this part of the experiment. Of the three products, the syndet was the least irritating and caused the least dryness, followed by the classic soap, with the irritant, basic soap causing the most redness and dryness.8
The results of this study emphasize the importance of choosing a cleanser with a pH that is close to the pH of the skin. The study also demonstrates that the more frequently a person uses a cleanser, the more side effects he is likely to experience. This is one of the reasons that you should only wash your skin with a cleanser a maximum of twice a day.
While these three studies focused on general side effects of soaps and cleansers, one study has looked specifically at how soaps and cleansers affect people with acne.
Study comparing effects of soap and syndet bar on acne

To date, only one study, published in 1995 in the journal Infection, has directly looked at the effects of how washing the face with different products affects acne. This study compared Lux Soap with one type of syndet bar, called Sebamed Kompakt. The participants of the study were 120 people between the ages of 14 and 24, all of whom suffered from mild to moderate acne. The researchers divided the participants into two groups, one of which used soap, and the other, the syndet bar, twice a day over a period of 12 weeks.9
In the group that used soap, the number of inflammatory and non-inflammatory acne lesions increased over the duration of the experiment. Twenty-three of these participants also experienced irritation from using soap. On the other hand, in the group that experimented with the syndet bar, the number of inflammatory and non-inflammatory lesions decreased, and only one person experienced irritation as a side effect.9
Although this study only compared one type of soap and one type of cleanser, the results confirm that people with acne should avoid using soap because it can irritate the skin and may worsen acne.

Based on the evidence, we recommend the following guidelines for choosing a soap or cleanser.
- Avoid soap. If you are not sure whether a product is soap, check the list of ingredients on the back. If any of the ingredients are found in the table of soap ingredients in this article, do not use the product.
- When choosing a cleanser, select the mildest one possible. You can check the list of ingredients on the back of the cleanser and consult the table of cleanser ingredients in this article. Avoid any ingredients that are labeled as “harsh” in the table and “medium” if they are within the first seven ingredients listed.
- Choose cleansers made specifically for the face.
- If possible, choose cleansers with a pH (acidity level) between 4 and 6. Unfortunately, the pH value usually is not provided on product labels, but pH testers are available at any drugstore and are easy to use. But don’t worry, most liquid facial cleansers that specifically state that they are “ultra-gentle” should be within this pH range.
- Select liquid cleansers rather than bars. This is important for 2 reasons. First, because the closed container helps prevent contamination by microbes (bacteria or mold). This is especially important if you are sharing the cleanser with other people. And second, research also suggests that the pH of liquid cleansers is closer to the pH of the skin compared to cleansing bars.6
- Look for terms like “mild,” “for sensitive skin,” and “hypoallergenic” on the label. While these terms are unregulated, they at least indicate the manufacturer is considering these things.
- Choose a fragrance-free cleanser whenever possible. Research shows that fragrances are a common cause of skin irritation, and it is important to avoid skin irritation if you are acne-prone.5 Look for a cleanser labeled “scent-free” or “fragrance-free.”
And remember, do not wash your skin more than two times a day. More than that can be irritating, and to get acne-prone skin clear, it is vital to keep it non-irritated.
References
- Solomon, B. A. & Shalita, A. R. Effects of detergents on acne. Clin Dermatol 14, 95 – 99 (1996). https://www.ncbi.nlm.nih.gov/pubmed/8901406
- Friedman, M. & Wolf, R. Chemistry of soaps and detergents: Various types of commercial products and their ingredients. Clin Dermatol 14, 7 – 13 (1996). https://www.ncbi.nlm.nih.gov/pubmed/8901393
- Mijaljica, D., Spada & F., Harrison, I. P. Skin cleansing without or with compromise: Soaps and syndets. Molecules 27, 2010-2025 (2022). https://pubmed.ncbi.nlm.nih.gov/35335373/
- Ananthapadmanabhan, K. P., Moore, D. J., Subramanyan, K., Misra, M. & Meyer, F. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther 17, 16 – 25 (2004). https://www.ncbi.nlm.nih.gov/pubmed/14728695
- Mukhopadhyay, P. Cleansers and their role in various dermatological disorders. Indian J Dermatol 56, 2-6 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21572782
- Pluetrattanabha, N., Kulthanan, K., Nuchkull, P. & Varothai, S. The pH of skin cleansers for acne. Indian J Dermatol Venereol Leprol 81, 181-185 (2015). https://pubmed.ncbi.nlm.nih.gov/25751340/
- Korting, H. C. & Braun-Falco, O. The effects of detergents on skin pH and its consequences. Clin Dermatol 14, 23 – 27 (1996). https://www.ncbi.nlm.nih.gov/pubmed/8901395
- Korting, H. C., Greiner, K., Hubner, K. & Hamm, G. Changes in skin pH and resident flora by washing with synthetic detergent preparations at pH 5.5 and 8.5. J Soc Cosmet Chem 42, 147 – 158 (1991). http://journal.scconline.org/abstracts/cc1991/cc042n03/p00147-p00158.html
- Baranda, L., Gonzalez-Amaro, R., Torres-Alvarez, B., Alvarez, C. & Ramirez, V. Correlation between pH and irritant effect of cleansers marketed for dry skin. Int J Dermatol 41, 494 – 499 (2002). https://www.ncbi.nlm.nih.gov/pubmed/12207765
- Barel, A. O., Lambrecht, R., Clarys, P., Morrison, B. M. & Paye, M. A comparative study of the effects on the skin of a classical bar soap and a syndet cleansing bar in normal use conditions and in the soap chamber test. Skin Res Technol 7, 98 – 104 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11393212
- Korting, H. C. et al. The influence of the regular use of soap or acidic syndet bar on pre-acne. Infection 23, 89 – 93 (1995). https://www.ncbi.nlm.nih.gov/pubmed/7622270
- Khosrowpour, Z., Ahmad Nasrollahi, S., Ayatollahi, A., Samadi, A. & Firooz, A. Effects of four soaps on skin trans-epidermal water loss and erythema index. J Cosmet Dermatol 18, 857-861 (2019). https://www.ncbi.nlm.nih.gov/pubmed/30160004
 Acne.org Products
Acne.org Products