Cyproterone Acetate Is an Anti-androgen Medication That Is Normally Found as Part of a Birth Control Pill and May Be Somewhat Effective Against Acne

The Essential Info
Cyproterone acetate (CPA) is an anti-androgen medication, which means it suppresses the production of androgens (male hormones that are found in both males and females) in the body. Because excessive levels of androgens contribute to acne, anti-androgen medications, such as CPA, can help treat acne.
Doctors prescribe CPA only for women when treating acne.
It can be taken alone, but it is almost always prescribed as part of a combined oral contraceptive (COC, or birth control pill). The brand names for this CPA-containing birth control pill are Diane-35® or Dianette®.
Several studies indicate that CPA-containing COCs help women achieve noticeable clearing of their skin, with studies revealing a 37%-90% reduction in acne. However, all COCs work to clear acne, not just those with CPA in them. Whether CPA-containing COCs work better remains an area of study.
Side Effects: Cyproterone acetate can cause side effects, such as menstrual irregularities, which are minor. In addition, CPA-containing COCs may raise the risk of a dangerous condition called venous thromboembolism (blood clot in the veins), though different studies have come to opposite conclusions on this topic, and the bulk of the research indicates that this risk is not higher in CPA-containing COCs than in most other ones. This possible risk of venous thromboembolism has caused controversy over CPA in several countries, with France banning CPA briefly in 2013.
Talk to Your Doctor: If you are a woman with acne that hasn’t responded to other treatments, and you also want to use birth control, a COC that contains CPA might be a treatment option for you. Keep in mind that any time you alter your hormones it is a serious decision, so be sure to talk to your doctor about the risks and benefits.

The Science
- CPA-containing Oral Contraceptives Are Effective in Treating Acne
- Side Effects of Cyproterone Acetate
- Controversy Surrounding Cyproterone Acetate
- The Bottom Line
Cyproterone acetate (CPA) is a progestin, which is a synthetic version of a female reproductive hormone called progesterone.

Doctors sometimes prescribe CPA to women to treat acne because it acts as an anti-androgen. Excessive levels of androgens (male hormones that are present in both males and females) often lead to an increase in skin oil production and acne. Therefore, reducing androgens can clear up the skin.1

As a 2014 article in Clinics in Dermatology states, “Androgens play an important role in [skin oil] production and excretion. This subsequently contributes to the formation of acne lesions.”2 Because anti-androgens reduce the amount of androgens circulating in the body, they can help clear up acne lesions.1
Cyproterone acetate can be taken alone, but when used for acne it is almost always prescribed in the form of a birth control pill (combined oral contraceptive – COC) that contains both CPA and an estrogen. In fact, researchers recommend that CPA by itself be prescribed only for women who no longer have a uterus or ovaries or who cannot tolerate estrogens.3
When taken alone, the recommended dose of CPA is 50 – 100 mg. This is significantly higher than the dose present in COCs, which is 2 mg. When taken alone, it should be started on either the first or fifth day of the menstrual cycle and stopped on the fourteenth day, just before ovulation. In contrast, COCs containing CPA should be taken daily.1
CPA-containing Oral Contraceptives Are Effective in Treating Acne
Multiple studies indicate that CPA-containing oral contraceptives are effective in treating acne, typically producing a 37-90% reduction in acne.4-6 There are no studies on CPA taken alone for acne.

Expand to read details of studies

A 2004 review in The European Journal of Contraception and Reproductive Health Care summarized the results of clinical trials that investigated the effectiveness of CPA in reducing acne. Here are the trials that were included in the review:
- A 1986 randomized, double-blind trial compared three different COCs. Double-blind means that neither the women nor the researchers knew which treatment the women received. Two of the COCs contained CPA: one had 35 micrograms of estrogen and 2 mg of CPA, and the other had 50 micrograms of estrogen and 2 mg of CPA. After six months of treatment, all of the women taking a CPA-containing COC experienced a 70 – 72% reduction in acne lesions. The difference in results between the two CPA-containing COCs was not significant, meaning that they worked about the same. The women taking the non-CPA COC experienced a 35% reduction in acne lesions. The review article did not mention whether the difference between the CPA-containing COCs and the other COC was significant.
- A 1988 double-blind trial compared a COC containing 35 micrograms of estrogen and 2 mg of CPA with a COC containing 50 micrograms of estrogen and 2 mg of CPA. After nine months, between 57% and 63% of the women experienced significant improvement in their acne. The difference between the results of the different COCs was not significant either.
- A 1990 randomized trial compared two different COCs, one of which contained CPA. After nine months, 81 – 86% of the women taking the CPA-containing COC experienced a significant improvement in their acne. In women taking the non-CPA COC, 63 – 80% experienced significant improvement. The review did not mention whether the difference between the CPA-containing COC and the non-CPA one was significant.
- A 1990 trial compared a COC containing 35 micrograms of estrogen and 2 mg of CPA with a COC containing 50 micrograms of estrogen and 2 mg of CPA. After 12 months, acne improved by 71% to 72%. The difference in results between the two COCs was negligible.
- A 1990 trial evaluated a COC containing 35 micrograms of estrogen and 2 mg of CPA in 890 women. After six months, 83% of the women experienced more than a 50% reduction in the number of acne lesions.
- A 1990 trial evaluated a COC containing 35 micrograms of estrogen and 2 mg of CPA (brand name: Diane-35) in 1161 women. After 36 months, 100% of the women experienced significantly improved acne of the face and chest, and 89% of the women experienced significant improvement of acne on the back.
- A 2002 randomized, double-blind trial compared a COC containing 35 micrograms of estrogen with another COC. In the 82 women taking the CPA-containing COC, the average acne lesion count decreased by 62.5% after nine months of treatment. The average lesion count in women taking the non-CPA COC decreased by 58.8%. The review did not mention whether the difference between the two COCs was significant.4

More recently, a 2009 randomized, double-blind trial published in Contraception compared the effectiveness of a COC that contained CPA, one that did not, and a placebo in women with mild-to-moderate acne. After six months of treatment with the CPA-containing COC, the average number of inflammatory acne lesions decreased by 64.6%, and the average number of total lesions decreased by 53.6%. Overall, 90.2% of the women taking the CPA-containing COC experienced improvement of their facial acne. This study also found that the CPA-containing COC worked about the same as the non-CPA COC and that both worked better than the placebo.5 Because this study also included data from multiple countries, the evidence is strong.
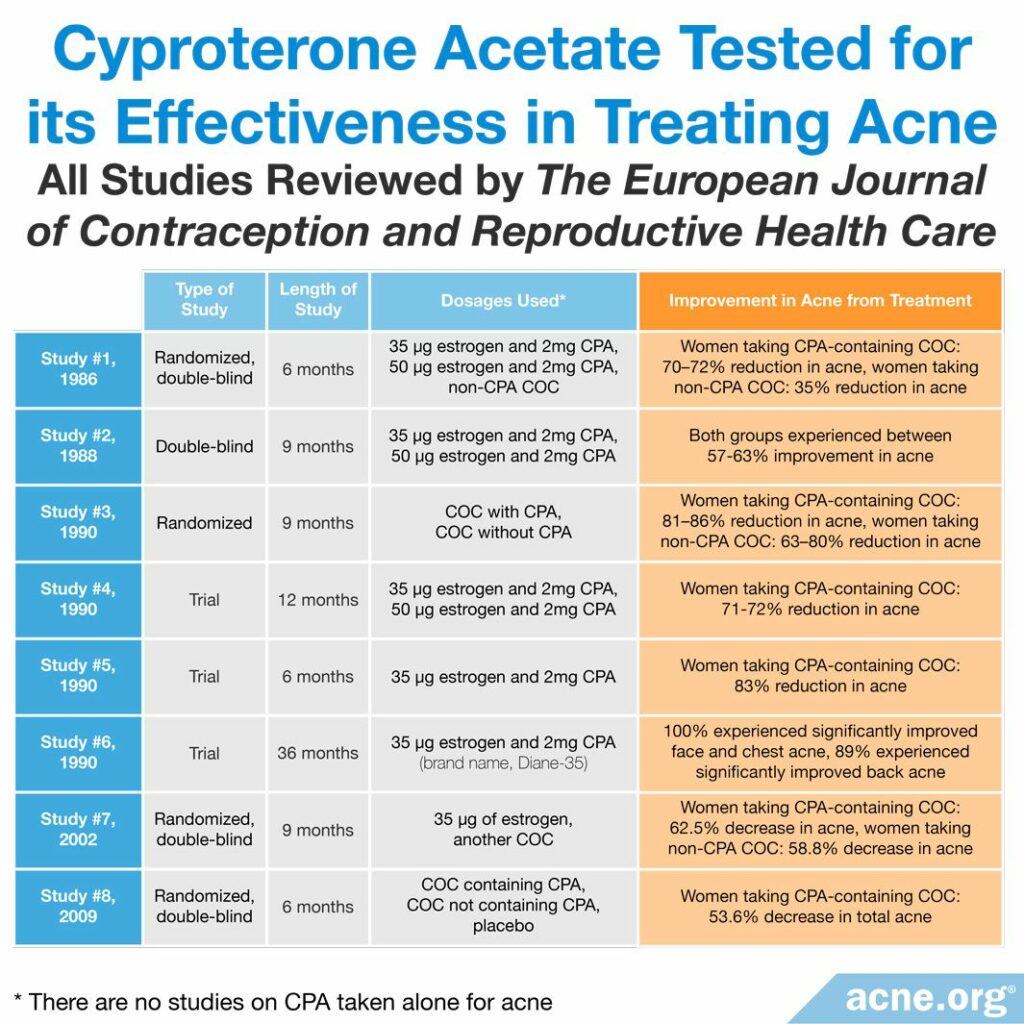
Overall improvement with CPA-containing COCs was between 37% and 90%. In addition, these studies suggest that improvement occurs slowly, over a period of several months.
All COCs are effective in reducing acne, whether they contain CPA or a different progestin.6 However, COCs containing CPA do tend to achieve better results in treating acne than COCs containing some other types of progestins. This was the conclusion reached by a systematic review published in 2012 in the Cochrane Database of Systematic Reviews.7

The authors of the review combed through all the studies published to date that had tested COCs as an acne treatment. They concluded that CPA-containing COCs seem to treat acne more effectively than COCs containing other progestins, such as levonorgestrel, desogestrel, or drospirenone. However, the authors of the review noted that some of the studies they looked at reported conflicting results.7
Therefore, what we can say with confidence at this point is that CPA-containing COCs do improve acne. The preponderance of the evidence so far also seems to suggest that COCs containing CPA may be superior to COCs containing other progestins.
We should also note that none of these studies tell us how much of the anti-acne effect might be due to the estrogen in the COCs. In other words, it might primarily be estrogen, and not CPA or other progestins, providing most of the medicinal benefit for women with acne. More studies are necessary to tease out the effect of CPA by itself.
Side Effects of Cyproterone Acetate
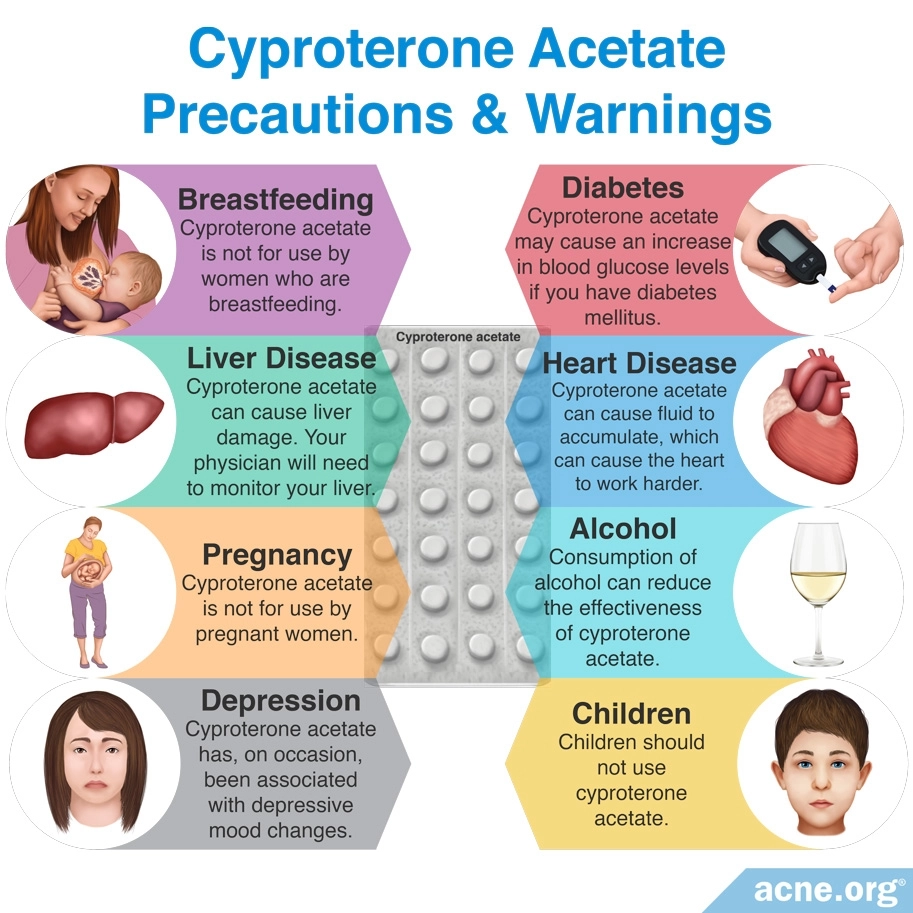
Cyproterone acetate can cause several side effects, including:
– Menstrual irregularities, such as bleeding more or less than normal and breakthrough bleeding (bleeding between menstrual periods)
– Breast tenderness
– Headache
– Nausea
All of these side effects tend to lessen or disappear over time. A less common but more serious side effect of CPA is liver toxicity, which is dose-dependent, meaning that it is more likely to occur with high doses.1
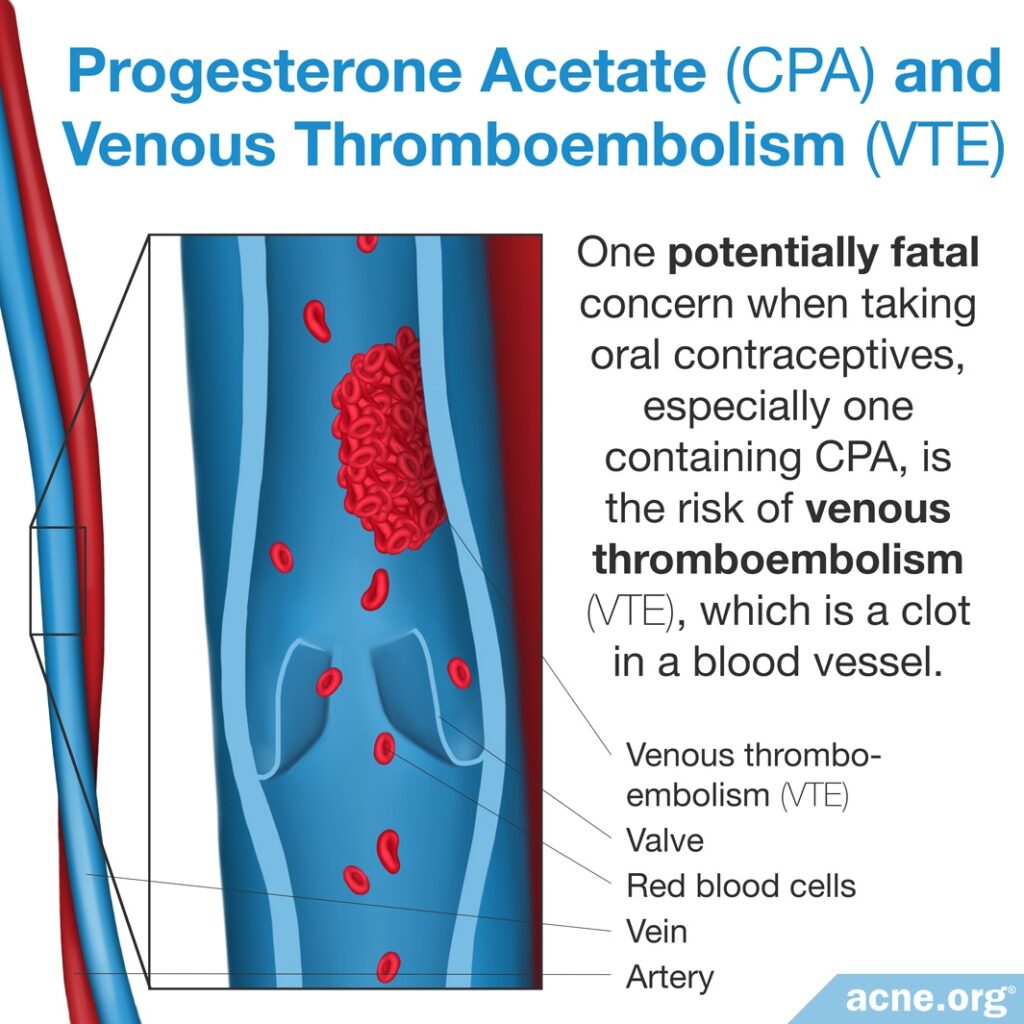
Venous Thromboembolism (VTE)
One serious concern when taking any COC, especially one that contains CPA, is the risk of venous thromboembolism (VTE), which is a clot in a blood vessel. Venous thromboembolism can result in serious health problems and even in death. Risk factors that increase the risk of VTE include older age, obesity, smoking, immobilization, such as long periods of bed rest or being in a cast, and some blood clotting diseases. The risk of VTE generally is low in young, healthy women, unless they are affected by any of these risk factors.2
Although scientists agree that all COCs come with the risk of VTE, recent research suggests that the estrogen component of the pill may determine how high that risk is. Currently, scientists recommend choosing a COC where the estrogen component is in the form of estradiol rather than ethinylestradiol. In other words, when it comes to choosing a CPA-containing COC, it might be best to look for a COC with CPA and estradiol as its active ingredients.8
Two studies report an increased risk of VTE when taking a COC that contains CPA.9,10 However, as we will see, other studies did not replicate these findings.
Expand to read details of studies

A 2001 study in The Lancet compared the risk of VTE in women taking a COC that contained CPA with the risk in women taking one that contained another progestin called levonorgestrel. The researchers concluded that the risk of VTE was four times higher in women taking a COC containing CPA.9

A 2003 study in Human Reproduction investigated the risk of VTE in women taking a COC that contained CPA. This study performed two different analyses. In the first, women taking the CPA-containing COC were at more than twice the risk of VTE compared to women taking a different COC. In the second analysis, women taking the CPA-containing COC had almost three times the risk of VTE compared to women taking a different COC. However, the authors noted that they could not exclude other factors that could have influenced their results.10
Two other studies found that the risk of VTE is not higher in CPA-containing COCs when compared to most other COCs.11,12
Expand to read details of studies

A 2004 study in Pharmacoepidemiology and Drug Safety used the same research-design as the 2003 study in Human Reproduction. The authors found that women taking a CPA-containing COC had twice the risk of VTE compared to women taking a different COC. However, this difference was not statistically significant, and they concluded that the risk of VTE is not higher for those taking CPA-containing COCs than for those taking a different one.11

A 2014 systematic review (a rigorous literature review that combines the results of many studies into a single analysis) in the Cochrane Database of Systematic Reviews evaluated the risk of VTE when taking a COC. This review included 26 studies and concluded that the risk of VTE is more than three times higher in people taking any COC than in those not taking one. However, CPA-containing COCs carried about the same risk as COCs containing other progestins, such as gestodene, desogestrel, and drospirenone. In addition, this review found that COCs containing the progestin, levonorgestrel, were associated with 20 – 50% lower risk of VTE than the others.12
We can conclude from these studies that the risk of VTE increases when taking a CPA-containing COC but that this risk probably is not any greater for CPA-containing COCs than for most other ones, except those containing levonorgestrel.
Controversy Surrounding Cyproterone Acetate

Despite the mixed research results, the possible increased risk of VTE in people taking a CPA-containing COC has caused controversy in several countries.
One scientific article describes many women switching from a CPA-containing COC called Diane-35 to a different COC following a television documentary which highlighted the possible risks of CPA.13 Another article describes a temporary ban on the sale of Diane-35 in France, which was afterwards lifted.14
Due to these controversies, the use of Diane-35 has decreased by 44 – 91% since 2011 in several European countries: the Netherlands, the United Kingdom, and Italy.15
Expand to read details of articles

According to a 2005 article in CMAJ, a Canadian television documentary on the CPA-containing COC called Diane-35 (known as Dianette in the UK) caused many women to switch from Diane-35 to a different COC. In February 2003, there was a sharp increase in the number of women, both with and without acne, who switched to a different COC.13

According to a 2013 article in BMJ, France banned the sale of Diane-35 in May 2013 because of the risk of VTE. Shortly thereafter, the European Commission ordered France to lift the ban, and the European Union required all European countries to follow the recommendations of the European Medicines Agency, which concluded that “the benefits of Diane-35 outweighed the risks, provided measures were taken to minimize those risks.”14 The European Medicines Agency concluded also that Diane-35 should be prescribed only to women who needed birth control and had moderate-to-severe acne or hirsutism (excess hair that grows in a male pattern, such as on the face) related to androgen sensitivity. In addition, the agency stated Diane-35 should be prescribed for acne only if all other treatments fail and that it should never be prescribed in combination with another hormonal birth control pill.14

A 2020 study published in the journal Contraception compared the use of Diane-35 from 2011 to 2017 in 3 European countries: the Netherlands, the United Kingdom, and Italy. The study looked at how many new users began taking this pill during each of those years. Compared to 2011, the number of new users taking Diane-35 in 2017 had plummeted by 91% in the Netherlands, by 44% in the United Kingdom, and by 50% in Italy.15
The Bottom Line

If you are a woman with acne that hasn’t responded to other treatments, and you also want to use a birth control pill, choosing a pill that contains CPA might be a treatment option for you. Be sure to talk to your doctor about the risks and benefits of this medication.
References
- Bettoli, V., Zauli, S. & Virgili, A. Is hormonal treatment still an option in acne today? Br. J. Dermatol. 172 suppl 1, 7 – 46 (2015). https://www.ncbi.nlm.nih.gov/pubmed/25627824
- Lam, C. L. & Zaenglein, A. L. Contraceptive use in acne. Clin. Dermatol. 32, 502 – 515 (2014). https://www.researchgate.net/publication/263857122_Contraceptive_use_in_acne
- Hammerstein, J., Meckies, J., Leo-Rossberg, I., Moltz, L. & Zielske, F. Use of cyproterone actetate (CPA) in the treatment of acne, hirsutism and virilism. J. Steroid Biochem. 6, 827 – 836 (1975). https://www.ncbi.nlm.nih.gov/pubmed/126335
- Del Marmol, V., Teichmann, A. & Gertsen, K. The role of combines oral contraceptives in the management of acne and seborrhea. Eur. J. Contracept. Reprod. Health Care 9, 107 – 124 (2004). https://www.ncbi.nlm.nih.gov/pubmed/15449823
- Palombo-Kinne, E., Schellschmidt, I., Schumacher, U. & Gräser, T. Efficacy of a combined oral contraceptive containing 0.030 mg ethinylestradiol/ 2 mg dienogest for the treatment of papulopustular acne in comparison with placebo and 0.035 mg ethinylestradiol/ 2 mg cyproterone acetate. Contraception 79, 282 – 289 (2009). https://www.ncbi.nlm.nih.gov/pubmed/19272497
- Kozłowski, M., Niedzielska, M., Lorenz, A., Brodowska, A., Malanowska, E., Przepiera, A., Cymbaluk-Płoska, A. & Sowińska-Przepiera, E. Metabolic and dietary factors in acne vulgaris and evaluation of the acne vulgaris treatment with oral contraceptive-based therapies in young adult women. Nutrients 15, 1488 – 1503 (2023). https://pubmed.ncbi.nlm.nih.gov/36986218/
- Arowojolu, A. O., Gallo, M. F., Lopez, L. M. & Grimes, D. A. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst. Rev. 7, CD004425 (2012). https://www.ncbi.nlm.nih.gov/pubmed/22786490
- Heikinheimo, O., Toffol, E., Partonen, T., But, A., Latvala, A. & Haukka, J. Systemic hormonal contraception and risk of venous thromboembolism. Acta Obstet. Gynecol. Scand. 101, 846-855 (2022). https://pubmed.ncbi.nlm.nih.gov/35633036/
- Vasilakis-Scaramozza, C. & Jick, H. Risk of venous thromboembolism with cyproterone or levonorgestrel contraceptives. Lancet 358, 1427 – 1429 (2001). https://www.ncbi.nlm.nih.gov/pubmed/11705493
- Seaman, H. E., de Vries, C. S. & Farmer, R. D. The risk of venous thromboembolism in women prescribed cyproterone acetate in combination with ethinyl estradiol: a nested cohort analysis and case-control study. Hum. Reprod. 18, 522 – 526 (2003). https://academic.oup.com/humrep/article/18/3/522/626070
- Seaman, H. E., de Vries, C. S. & Farmer, R. D. Venous thromboembolism associated with cyproterane acetate in combination with ethinyloestradiol (Dianette): observational studies using the UK General Practice Research Database. Pharmacoepidemiolog. Drug Saf. 13, 427 – 436 (2004). https://www.ncbi.nlm.nih.gov/pubmed/15269926
- De Bastos, M. et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst. Rev. 3, CD010813 (2014). https://www.ncbi.nlm.nih.gov/pubmed/24590565
- Mintzes, B., Morgan, S. & Bassett, K. L. Medicine by media: did a critical television documentary affect the prescribing of cyproterone-estradiol (Diane-35)? CMAJ 173, 1313 – 1315 (2005). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1283492/
- Arie, S. European Commission orders France to lift ban on acne pill. BMJ 347, f4932 (2013). https://www.bmj.com/content/347/bmj.f4932
- Penning-van Beest, F. J. A., Bezemer, I. D., Smits, E., García Rodríguez, L. A., Cea Soriano, L., Lapi, F., Simonetti, M., Asiimwe, A. & Herings, R. M. C. Reduction in use of cyproterone/ethinylestradiol (Diane-35 and generics) after risk minimization measures in the Netherlands, UK and Italy. Contraception 102, 243-245 (2020). https://pubmed.ncbi.nlm.nih.gov/32470466/
 Acne.org Products
Acne.org Products