That Pharmaxis product doesn't look like it actually works well.
https://company-announcements.afr.com/asx/pxs/1c1487d4-b080-11ec-9922-32a0664dadc9.pdf
Some of the controls look better than when the compoundwas used.
Verteporfin is potentially the most successful scar healing option Ive seen. I would really like to hear preliminary results from those who are currently testing it.
There is a telegram of people researching and self experimenting with verteporfin for scars and hair. You can join here: [Removed]
man, it's just so frustrating how subpar the avaliable treatment options are
Big News!
New study on Verteporfin scarless healing just came out with good results
https://drive.google.com/file/d/1qDEmwaM7CnsJ2NpMAPIO_Cwas_wW19I4/view?usp=sharing
"In summary, our study clearly demonstrated that YAP/TAZ
are highly expressed and undergo increased nuclear transloca-
tion in keloid fibroblasts. We also demonstrated that inhibition
of YAP/TAZ via either gene knockdown or verteporfin treatment
significantly inhibits fibroblast proliferation, induced fibroblast
apoptosis and downregulates the COL1A1 production of keloid
fibroblasts. Thus, we provide evidence that YAP/TAZ may be in-
volved in the pathogenesis of keloids. Although verteporfin has
limited clinical use as a YAP/TAZ inhibitor due to its photosensi-
tive and proteotoxic effects,11 our research suggests that inhibi-
tion of YAP/TAZ overall may be an innovative target for treating
keloids."
Btw this study was preformed on human tissue
On 3/22/2022 at 6:53 PM, ScarfromtheLionKing said:Lots of bad news.
1. Verteporfin apparently doesn't work. This plastic surgeon says it's not helpful. I understand it hasn't been through all the clinical trials yet, but since he is a plastic surgeon I'm guessing he has tried the drug and found it to be ineffective. I'm really, really hoping that he's just wrong or dishonest, but I think that view is irrational. It's in the post "Scar revision with off-label use of Verteporfin?" on Realself: https://www.realself.com/question/los-angeles-california-tats-unis-scar-revision-label-verteporfin
2. Microcoring is not being tested for acne scars. I talked to Cytrellis, the company that owns Ellacor, and they said they had no plans for doing trials with acne scars. Then again, there was that clinical trial posted ^above that says it's being tested for skin regeneration. So maybe they just meant it wasn't being tested for acne scars specifically? Maybe they were misinformed? Or maybe it is being tested by a company other than Cytrellis?
If he has actually tested Verteporfin on patients without any human trails he probably isn't a surgeon you should be listening to. The dosage and timing matters and it's unlikely that he actually tested it. He is just trying to get people to buy his treatments now and not wait for a cure in the near future.
It also doesn't help he gave no explanation why it doesn't work and just went to promoting his treatments directly after.
46 minutes ago, Candy Says said:@presnwhere did you get that article? Some website, medical magazine, database or what? Want to subscribe to whatever it is.
It's from the journal experimental dermatology https://onlinelibrary.wiley.com/journal/16000625 . However the full PDF of the study that I provided you would need to pay for...
Have some optimism verteporfin now has a study that shows it can prevent fibrosis in human tissue which is huge
Furthermore there is another study that will come out shortly that determined verteporfin can prevent scaring in human lung tissue also https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A5226 . The abstract has just recently been posted.
"Conclusion: Therapeutic targeting of the YAP/TAZ pathway using an FDA approved drug resulted in attenuated fibrosis in an in vivo mouse and in the ex vivo human tissue-based FC-PCLS model. Targeting of YAP/TAZ using VP may represent a new therapeutic strategy for IPF."
@Candy Saysforgot to mention this study that is also going to come out soon
IPF isIdiopathic pulmonary fibrosis btw (scarring of the lung tissue)
On 5/8/2022 at 7:45 AM, Skin Pessimist said:man, it's just so frustrating how subpar the avaliable treatment options are
I have scars for a long time more than 10 years and they do look better than before even though all the scars are still there. You won't have 100% improvement but you can make the scars smoother and less prominent with time.
After so long I kind of made peace with myself. If scarless healing happens it happens if it doesn't it doesn't. I just accept my scars as fated by god. I have to live my life regardless.
Well dermal md scar serum was recommended by my Doctor to help with scar healing and it has done a great job so far. A little pricey for the size but then again you need to use very little. I've used it twice a day and was told to use it for 6 weeks for the scaring to completely heal.
Early peaks about pig study from Stanford things look promising.
13 hours ago, NagarNikku_ said:This is HUGE.
It is pretty cool huh.
Still ways away though so we shouldnt put all our emotional eggs in the vert basket, anything can happen. Just remember to judge each study objectively and not think that just cause every past scar treatment research failed that this one will too, theyre all independent events.
In all my time researching this topic, I think this is the first time that a true candidate has appeared, and is gaining traction. Regardless of whether it fails or succeeds, its a pretty exciting time for the scarless healing enthusiasts.
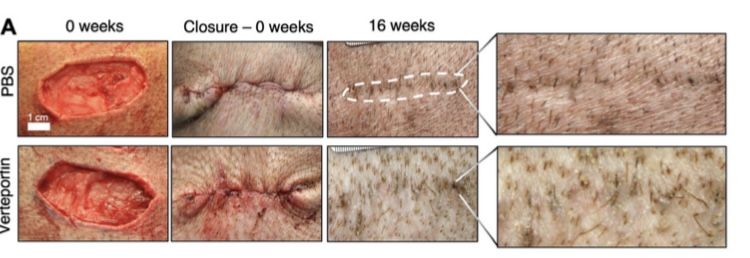
It is clear continuation of what was found in mice study. I can see only a bit of color problems, but not scar tissue.
Looks like it's the first success in the field of scarless healing. Finally, at least something. I guess...
Also, pay attention to how wide and ugly was the wound (i mean, it's not just a straight cut).
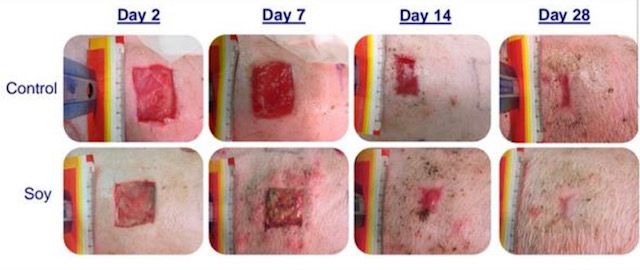
Some more news. A Soy-protein matrix skin scaffold that leaves little to no scaring is going to be released in 6 months. I have asked the CEO and he confirmed. Here is the link to the product. https://www.neuesse.com/skin-conditions/post-laser-ablation-skin-resurfacing/
Also here is a description of the product:
OmegaSkin has been proven to heal full thickness wounds with minimal to no scarring. Further in a patient™s favor is the healed wound will have fully functioning hair follicles and sweat glands. Thus OmegaSkin œHeals like the Skin You™re in.
We have a robust pipeline of products which includes liquid sprayable skin and a proprietary handheld electrospinner for delivery. NeuEsse is developing the portable spinner to deliver other products for aging and traumatized skin such as wrinkle and rejuvenation compounds and will be prominent in Dermatology. The application device is designed to reduce application pain in wound patients where direct manual treatments can be painful.
Our OmegaSkin„ will provide off-the-shelf solutions in pre-manufactured sheets and a handheld portable device for no-touch applications of our Liquid Skin and other commercial therapeutics.
7 hours ago, presn said:Some more news. A Soy-protein matrix skin scaffold that leaves little to no scaring is going to be released in 6 months. I have asked the CEO and he confirmed. Here is the link to the product. https://www.neuesse.com/skin-conditions/post-laser-ablation-skin-resurfacing/
Also here is a description of the product:
OmegaSkin has been proven to heal full thickness wounds with minimal to no scarring. Further in a patient™s favor is the healed wound will have fully functioning hair follicles and sweat glands. Thus OmegaSkin œHeals like the Skin You™re in.
We have a robust pipeline of products which includes liquid sprayable skin and a proprietary handheld electrospinner for delivery. NeuEsse is developing the portable spinner to deliver other products for aging and traumatized skin such as wrinkle and rejuvenation compounds and will be prominent in Dermatology. The application device is designed to reduce application pain in wound patients where direct manual treatments can be painful.
Our OmegaSkin„ will provide off-the-shelf solutions in pre-manufactured sheets and a handheld portable device for no-touch applications of our Liquid Skin and other commercial therapeutics.
and when it comes to the market?
I already mentioned it. 6 months from now @Diamond9199. Considering they are partnered with CMU (one of the top universities in the U.S. https://engineering.cmu.edu/pita/projects/public-health-medicine/2020/soy-protein-based-campbell.html ) they are probably telling the truth.
On 2/15/2022 at 6:43 AM, lehran said:Been a long time since I came here fellas:
About Elastagen: the reason you're not hearing about anything is because of a NDA. Elastagen was first acquired by Allergan (a big pharmaceutical company) which was afterwards acquired by Abbvie (one of the biggest pharmaceutical companies in the world)...sooooo basically yes, it is in the pipeline obviously, they're working on it in their labs, but you're not going to hear about anything regarding it until Abbvie chooses to do so.
It's good news...but it's frustrating: it's good in the sense that it's really going to happen, but it's frustrating in the sense that we have very little ways to find out anything else about it.
Here is proof that Elastagen is in the pipeline. Allergan filed this patent on the use of Tropoelastin for acne scars. This also has the results of the study conducted in depth! There are even more patents on Tropoelastin from Allergan you just have to look them up.
https://patents.google.com/patent/WO2021037733A1/en
Here is another scarless hydrogel company that is really promising coming from the University of Toronto. Unlike Sunogel, they have been giving frequent updates (last one is may 2022) and have investment from TWO major firms. In a 2020 interview, they claimed that it will be available for surgical use in 2-3 YEARS (The pandemic likely slowed this down a bit; although they just have been designated as a type VI medical device in Canada). This one is legit and likely coming very soon. I will keep you updated when the company representative responds to an email with questions.
"About Quthero:
Quthero aims to disrupt skin care following dermatological procedures such as laser skin resurfacing & microneedling and to eliminate scarring post plastic surgery using patented hydrogels. Quthero™s flagship hydrogel product enables full regrowth of human skin 60-200% faster than current products and without scarring. At the heart of all Quthero™s products is a unique peptide sequence, discovered and developed at MIT, Boston Children™s Hospital and University of Toronto, that is demonstrated to promote full and scarless skin regeneration. This unique molecule is incorporated into three new products launched
through Quthero Skincare, targeting cosmetic dermatology"
@presn I am here to appreciate your contribution to this thread. Very interesting stuff.
 Acne.org Products
Acne.org Products