1 hour ago, nikkigirl said:Scarring isn't on the list of the world's problems....maybe for us though. The company can't just release without a lot of testing. It might get rid of scarring but 10 years down the line it might cause skin cancer or other side effects. CollegeKidd...you are getting your hopes up too high only to get probably a let down. We have had laser,isologen,fillers ect that have not done much for scarring. I have just not seen any results yet..until then i am a skeptic.
I had made a lengthier post that didn't save, but the gist was this...
While it's healthy to have some expectancy and to be realistic, it's also unwise to bring up things that won't be true of the products CollegeKidd mentioned. Neither will cause cancer 10 years down the line. In SkinTE's case it's just using your own cells to guide the wound bed toward regeneration. In the case of Sunogel, they're using a degradable hydrogel that is absorbed. Nothing is added like stem cells, gene therapy or any of the other things going on with other research that would cause concern for adverse reactions.
And CollegeKidd, remember all the good things that have happened this year! A world's first with complete regeneration in pigs, SkinTE is in clinics now (eighteen if memory serves) and they have a pilot clinical trial going concurrently. Sunogel is working toward the same and Dr. Sun, in communications with posters on this site, has repeatedly stated that he believes the product will work in humans across all types of scars. Granted, both have raised concerns about the invasiveness of the procedures for the types of scars most suffer from on this site.
Both of these weren't even companies before 2016! That's pretty awesome progress in just one year and 2018 could hold even bigger things. Obviously, await the results and be shown that they've fulfilled their burden, but don't despair either.
20 hours ago, golfpanther said:I had made a lengthier post that didn't save, but the gist was this...While it's healthy to have some expectancy and to be realistic, it's also unwise to bring up things that won't be true of the products CollegeKidd mentioned. Neither will cause cancer 10 years down the line. In SkinTE's case it's just using your own cells to guide the wound bed toward regeneration. In the case of Sunogel, they're using a degradable hydrogel that is absorbed. Nothing is added like stem cells, gene therapy or any of the other things going on with other research that would cause concern for adverse reactions.
And CollegeKidd, remember all the good things that have happened this year! A world's first with complete regeneration in pigs, SkinTE is in clinics now (eighteen if memory serves) and they have a pilot clinical trial going concurrently. Sunogel is working toward the same and Dr. Sun, in communications with posters on this site, has repeatedly stated that he believes the product will work in humans across all types of scars. Granted, both have raised concerns about the invasiveness of the procedures for the types of scars most suffer from on this site.
Both of these weren't even companies before 2016! That's pretty awesome progress in just one year and 2018 could hold even bigger things. Obviously, await the results and be shown that they've fulfilled their burden, but don't despair either.
Seeing is believing............I hope you are right but companies always promise the world and fail to deliver.Remember Isologen?
Perhaps a silly question but one that still warrants clarification. Do these miracle drugs in the pipeline work for NEW scars (eg. fresh wound from surgery, trauma, etc) or for EXISTING scars (acne scars from years ago)? As you may know, preventing new scars from forming is not the same as getting rid of the old scars, which is what most of us who congregate here are interested in.
1 hour ago, nikkigirl said:22 hours ago, golfpanther said:I had made a lengthier post that didn't save, but the gist was this...While it's healthy to have some expectancy and to be realistic, it's also unwise to bring up things that won't be true of the products CollegeKidd mentioned. Neither will cause cancer 10 years down the line. In SkinTE's case it's just using your own cells to guide the wound bed toward regeneration. In the case of Sunogel, they're using a degradable hydrogel that is absorbed. Nothing is added like stem cells, gene therapy or any of the other things going on with other research that would cause concern for adverse reactions.
And CollegeKidd, remember all the good things that have happened this year! A world's first with complete regeneration in pigs, SkinTE is in clinics now (eighteen if memory serves) and they have a pilot clinical trial going concurrently. Sunogel is working toward the same and Dr. Sun, in communications with posters on this site, has repeatedly stated that he believes the product will work in humans across all types of scars. Granted, both have raised concerns about the invasiveness of the procedures for the types of scars most suffer from on this site.
Both of these weren't even companies before 2016! That's pretty awesome progress in just one year and 2018 could hold even bigger things. Obviously, await the results and be shown that they've fulfilled their burden, but don't despair either.
Seeing is believing............I hope you are right but companies always promise the world and fail to deliver.Remember Isologen?
They were not as far along in the whole regeneration world when isolagen came out. That was a different time. The whole stem cell regeneration world is exploding
6 hours ago, Sirius Lee said:Perhaps a silly question but one that still warrants clarification. Do these miracle drugs in the pipeline work for NEW scars (eg. fresh wound from surgery, trauma, etc) or for EXISTING scars (acne scars from years ago)? As you may know, preventing new scars from forming is not the same as getting rid of the old scars, which is what most of us who congregate here are interested in.
Not a silly question at all! But in both Polarity and Sunogel's case this doesn't matter because of how they're applied.
Why? Because both excise the scar tissue if it exists and create a brand new wound bed for the product's application. If you cut out a scar then there is no more scar, only a wound bedsame as if you had a fresh wound that was deep enough to form one.
Once the wound bed is there both SkinTE and Sunogel would guy the body's healing toward regeneration instead of scarring. This is how it worked on the pigs in their preclinical tests and how they've both said it is designed to work in humans.
7 hours ago, nikkigirl said:On 12/2/2017 at 7:18 PM, golfpanther said:I had made a lengthier post that didn't save, but the gist was this...While it's healthy to have some expectancy and to be realistic, it's also unwise to bring up things that won't be true of the products CollegeKidd mentioned. Neither will cause cancer 10 years down the line. In SkinTE's case it's just using your own cells to guide the wound bed toward regeneration. In the case of Sunogel, they're using a degradable hydrogel that is absorbed. Nothing is added like stem cells, gene therapy or any of the other things going on with other research that would cause concern for adverse reactions.
And CollegeKidd, remember all the good things that have happened this year! A world's first with complete regeneration in pigs, SkinTE is in clinics now (eighteen if memory serves) and they have a pilot clinical trial going concurrently. Sunogel is working toward the same and Dr. Sun, in communications with posters on this site, has repeatedly stated that he believes the product will work in humans across all types of scars. Granted, both have raised concerns about the invasiveness of the procedures for the types of scars most suffer from on this site.
Both of these weren't even companies before 2016! That's pretty awesome progress in just one year and 2018 could hold even bigger things. Obviously, await the results and be shown that they've fulfilled their burden, but don't despair either.
Seeing is believing............I hope you are right but companies always promise the world and fail to deliver.Remember Isologen?
Isologen was an injection made into existing scar tissue, wrinkles or other skin defects that was designed to rejuvenate skin, not provide complete regeneration. They were charlatans selling marginal benefits that are hard to define just like Juvista.
Polarity and Sunogel's aims and methods are completely different. They're not injections, but rather invasive procedures. As stated earlier in this post, they excise the entire scar in order to create a wound bed. In every study performed on regenerating skin tissues, be at by Polarity and Sunogel or someone else, the creation of wound bed is critical to regeneration.
From there the products are applied with a dressing over them and both aim to guide the body's natural healing process toward a regenerative path.
The fact that both Sunogel and PolarityTE have gotten complete regeneration in pigs is a huge deal. No, it isn't humans, but it puts them farther than anyone else has come and they both have stated their expectation that it will work on humans. In the case of SkinTE it shouldn't be long before we see results.
6 minutes ago, golfpanther said:Not a silly question at all! But in both Polarity and Sunogel's case this doesn't matter because of how they're applied.
Why? Because both excise the scar tissue if it exists and create a brand new wound bed for the product's application. If you cut out a scar then there is no more scar, only a wound bedsame as if you had a fresh wound that was deep enough to form one.
Once the wound bed is there both SkinTE and Sunogel would guy the body's healing toward regeneration instead of scarring. This is how it worked on the pigs in their preclinical tests and how they've both said it is designed to work in humans.
But how would this apply to acne scars, particularly if you have extensive acne pits all over your face? Surely, you can't just sand down the entire face before applying their products.
9 hours ago, Sirius Lee said:But how would this apply to acne scars, particularly if you have extensive acne pits all over your face? Surely, you can't just sand down the entire face before applying their products.
JohnRottenSkin's post of the convo Tiano had with Swanson pretty much covers this. As Swanson states, they're working on derivative products for more cosmetic leaning application, but believe SkinTE would work as is but be too invasive for most scars. If you did use SkinTE for acne, I'd imagine the surgeon would want to go piece by piece instead of excising or debriding it all at once. That's speculation on my part of course.
For Sunogel, I'm not sure. Dr. Sun, in conversations with others on this site, has brought up the invasiveness of the procedure but believes it will work in all cases. Sunogel is slightly less invasive because it doesn't need a biopsy site of healthy tissue.
12 hours ago, JohnRottenSkin said:Interview with Dr. Swanson, M.D. COO and Vice President of PolarityTEDr. Swanson gave me a detailed answer that all 3 layers can be excised whether in wounds, burns or scars for SkinTE to then be applied. He added that partial excisions can be made as well whether the wound just extends to the dermal layer of the skin or even just PART of the dermis.He went into more detail on how the hypodermis or subcutaneous tissue can be left alone if it's not damaged/injured and made it clear to me that the full dermis does NOTneed to be excised if it is not necessary further stating that the lower (reticular) dermis can be left alone while excising the upper (papillary) dermis only if appropriate.3.)Tano1:"We see also that you plan to branch out into cosmetic/scar revisions if successful with burns. Now assuming that SkinTE succeeded in burn wounds (and of course we believe its going to), will this be a viable solution for skin conditions and/or defects such as: acne scars, surgical scars, hypo-hyperpigmentation or even damage that has been seen from currently utilized skin and scar treatments? just to name a few."
Dr. Swanson:"Yea you know, it's hard to give the exact answer on every single one of those indications. Some of those could potentially be addressed with the SkinTE product as it stands today and others we are in the process of developing derivative products using similar technology to address." "It's a lot of nuances to what would be best in each situation, but you know I think that old scars, large scars can certainly be removed and have SkinTE applied to the base of the wound if the provider and patient think that could be beneficial."
(Note: Bold colored texts above are mine)
Thanks for the transcript. Sounds promising. But it sounds very invasive. This would be especially difficult (if not cumbersome) for acne scar sufferers considering that they would need to literally excise the scars on their face. As I already stated in my previous post, this would be a real challenge if you have a lot of scars all over your face. Also this type of treatment would likely require inpatient care and longer recovery downtime.
4 hours ago, golfpanther said:JohnRottenSkin's post of the convo Tiano had with Swanson pretty much covers this. As Swanson states, they're working on derivative products for more cosmetic leaning application, but believe SkinTE would work as is but be too invasive for most scars. If you did use SkinTE for acne, I'd imagine the surgeon would want to go piece by piece instead of excising or debriding it all at once. That's speculation on my part of course.For Sunogel, I'm not sure. Dr. Sun, in conversations with others on this site, has brought up the invasiveness of the procedure but believes it will work in all cases. Sunogel is slightly less invasive because it doesn't need a biopsy site of healthy tissue.
I guess it's better than what we have in store at the moment, but it seems to be too invasive--especially for those with a ton of scars. One at a time would be like a death by a 1000 papercuts. Ggrrrr!
22 minutes ago, Sirius Lee said:(Note: Bold colored texts above are mine)Thanks for the transcript. Sounds promising. But it sounds very invasive. This would be especially difficult (if not cumbersome) for acne scar sufferers considering that they would need to literally excise the scars on their face. As I already stated in my previous post, this would be a real challenge if you have a lot of scars all over your face. Also this type of treatment would likely require inpatient care and longer recovery downtime.
I guess it's better than what we have in store at the moment, but it seems to be too invasive--especially for those with a ton of scars. One at a time would be like a death by a 1000 papercuts. Ggrrrr!
Keep in mind that elsewhere in that transcript Swanson talks about how they're developing derivative products of SkinTE that are more geared for cosmetic applications. They also answer a similar question in the investor call they have posted on their website.
As of now though, even with derivative products it's likely that excision will be necessary to get complete regeneration. A wound bed, as I stated before, is critical in terms of getting the desired results (i.e. regeneration with skin appendages).
Do you guys think they don't want to treat patients with acne scars with SkinTe because excision to correct acne scars is too invasive?
That would be really stupid.....I have my scars mainly in my back and I wouldn't mind to get my skin excised in my back, but even in my face I wouldn't care too much if it would get rid of my acne scars forever.
I just can't accept they don't even attempt to treat acne sufferers with SkinTE ![]()
I would bet their derivative product purposefully developed for acne scars won't be out for many years....I can't wait that much
Maybe we should try to convince them in some way....like sending emails, calls, gathering firms or whatever.
We literally could have a cure for our problem and we're denied it.
And I'd bet the overwhelming majority of acne scars sufferers (even the ones that post on this forum) don't even know a product like SkinTE exist and waste their time and money with useless treatments like lasers, peeling, infini, needling, fillers and whatever....
If all of us send emails to them, they at least should rethink about it.
1 hour ago, SimpleMutton said:Do you guys think they don't want to treat patients with acne scars with SkinTe because excision to correct acne scars is too invasive?
That would be really stupid.....I have my scars mainly in my back and I wouldn't mind to get my skin excised in my back, but even in my face I wouldn't care too much if it would get rid of my acne scars forever.I just can't accept they don't even attempt to treat acne sufferers with SkinTE
I would bet their derivative product purposefully developed for acne scars won't be out for many years....I can't wait that muchMaybe we should try to convince them in some way....like sending emails, calls, gathering firms or whatever.
We literally could have a cure for our problem and we're denied it.
And I'd bet the overwhelming majority of acne scars sufferers (even the ones that post on this forum) don't even know a product like SkinTE exist and waste their time and money with useless treatments like lasers, peeling, infini, needling, fillers and whatever....
If all of us send emails to them, they at least should rethink about it.
To be clear, Swanson never said SkinTE couldn't be used on acne scars or other non-burn scars, just that the procedure might be too invasive for most surgeons to perform it. Remember, it's not up to Swanson if it gets used in a certain way or not, it's up to whomever you find to actually do the procedure.
He did say that if the surgeon and patient thought the invasiveness was worth it then he believes, based on the research they've done to this point, it would provide the same regeneration. I'm sure if you look hard enough you could find a surgeon and derm willing to do it if it works in humans.
For those who are in the dark, It's a real bitch to get FDA approval in the USA. Most do not even make to the phase 3. Here's a quick rundown:
Steps from Test Tube to New Drug Application Review
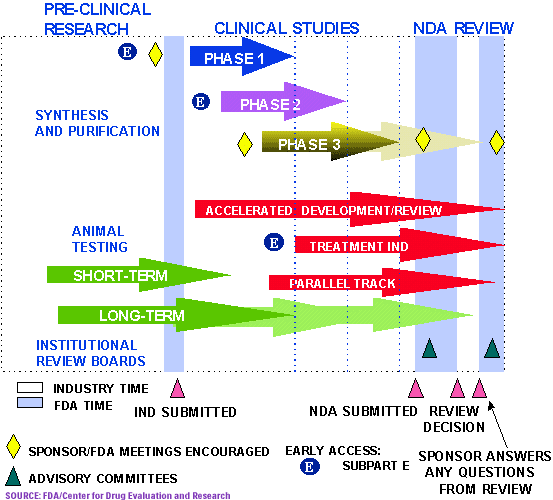
It takes on average 12 years and over US$350 million to get a new drug from the laboratory onto the pharmacy shelf. Once a company develops a drug, it undergoes around three and a half years of laboratory testing, before an application is made to the U.S. Food and Drug Administration (FDA) to begin testing the drug in humans. Only one in 1000 of the compounds that enter laboratory testing will ever make it to human testing.
If the FDA gives the green light, the "investigative" drug will then enter three phases of clinical trials:
- Phase 1 uses 20-80 healthy volunteers to establish a drug's safety and profile. (about 1 year)
- Phase 2 employs 100-300 patient volunteers to assess the drug's effectiveness. (about 2 years)
- Phase 3 involves 1000-3000 patients in clinics and hospitals who are monitored carefully to determine effectiveness and identify adverse reactions. (about 3 years)
Has this article been posted here?
Regeneration of the entire human epidermis using transgenic stem cells
Junctional epidermolysis bullosa (JEB) is a severe and often lethal genetic disease caused by mutations in genes encoding the basement membrane component laminin-332. Surviving patients with JEB develop chronic wounds to the skin and mucosa, which impair their quality of life and lead to skin cancer. Here we show that autologous transgenic keratinocyte cultures regenerated an entire, fully functional epidermis on a seven-year-old child suffering from a devastating, life- threatening form of JEB. The proviral integration pattern was maintained in vivo and epidermal renewal did not cause any clonal selection. Clonal tracing showed that the human epidermis is sustained not by equipotent progenitors, but by a limited number of long-lived stem cells, detected as holoclones, that can extensively self-renew in vitro and in vivo and produce progenitors that replenish terminally differentiated keratinocytes. This study provides a blueprint that can be applied to other stem cell-mediated combined ex vivo cell and gene therapies.
This major clinical development was based on decades of basic research. The clinical data gathered during 21 months of follow-up after the boy™s treatment have also led to major insights into human skin biology, as discussed in an accompanying News & Views (M. Aragona and C. Blanpain Nature http://dx.doi.org/10.1038/nature24753; 2017). For example, normal regeneration of the epidermis is directed by only a few stem-cell clones that can self-renew.
By their nature, highly personalized treatments using gene therapies and products derived from an individual™s stem cells are likely to be applicable to only a subset of patients. Although the report presents the treatment of one patient, it is a classic case of researchers standing on the shoulders of others. This project, for example, relied on long-term follow-up of a patient treated in 2006, as well as parallel studies that underpinned the development of tools for ex vivo gene therapy and for growing transplantable sheets of epidermis in vitro.
17 hours ago, Sirius Lee said:For those who are in the dark, It's a real bitch to get FDA approval in the USA. Most do not even make to the phase 3. Here's a quick rundown:
Steps from Test Tube to New Drug Application Review
It takes on average 12 years and over US$350 million to get a new drug from the laboratory onto the pharmacy shelf. Once a company develops a drug, it undergoes around three and a half years of laboratory testing, before an application is made to the U.S. Food and Drug Administration (FDA) to begin testing the drug in humans. Only one in 1000 of the compounds that enter laboratory testing will ever make it to human testing.
If the FDA gives the green light, the "investigative" drug will then enter three phases of clinical trials:
- Phase 1 uses 20-80 healthy volunteers to establish a drug's safety and profile. (about 1 year)
- Phase 2 employs 100-300 patient volunteers to assess the drug's effectiveness. (about 2 years)
- Phase 3 involves 1000-3000 patients in clinics and hospitals who are monitored carefully to determine effectiveness and identify adverse reactions. (about 3 years)
Only after successful phase 3 can the drug be released to the public!!!
As John stated, SkinTE is already approved and in clinics as I type this. It™s likely already been used on some humans at this point; either as part of the pilot clinical trial or as a product. We just haven™t heard about it because as a publicly traded company they™ll want to release results when it™s most advantageous for them and their shareholders.
Neither Sunogel or SkinTE need to go to phase 3 because they are considered devices. Why? Because nothing is added (ie stem cells, drugs etc) to them that would classify them as a drug.
The other post you made recently would need phase 3 because they™re putting induced stem cells into the body.
lastly, the FDA recently changed their regulatory process for regenerative medicine to get products to patients faster.
Dr Speigal a cranial facial reconstructive plastic surgeon says 10 years because he does a lot of cutting and his job is to hide the scarring. He said it will be at least 10 years before something that works will be on the market. I hope they have something on the market faster but like always people release treatments that don't work to get your money.
9 minutes ago, nikkigirl said:Dr Speigal a cranial facial reconstructive plastic surgeon says 10 years because he does a lot of cutting and his job is to hide the scarring. He said it will be at least 10 years before something that works will be on the market. I hope they have something on the market faster but like always people release treatments that don't work to get your money.
he knows nothing
50 minutes ago, nikkigirl said:Dr Speigal a cranial facial reconstructive plastic surgeon says 10 years because he does a lot of cutting and his job is to hide the scarring. He said it will be at least 10 years before something that works will be on the market. I hope they have something on the market faster but like always people release treatments that don't work to get your money.
Thank you for yet another insightful comment.
On 12/6/2017 at 2:57 PM, nikkigirl said:Dr Speigal a cranial facial reconstructive plastic surgeon says 10 years because he does a lot of cutting and his job is to hide the scarring. He said it will be at least 10 years before something that works will be on the market. I hope they have something on the market faster but like always people release treatments that don't work to get your money.
He may end up being right, but keep in mind, he's a surgeon and not a scientist/doctor working on scar free healing and regenerative medicine. Those are two dramatically different things.
I'm sure he knows a lot about how to properly perform a surgery to minimize or hide scarring. Does that mean he knows about all the intricacies of wound healing like Dr. Sun or PolarityTE does? No, and he likely doesn't know about the existence of either product because in my experience, a lot of derms and surgeons are too busy focusing on their practice to have time to research disruptive medicine.
Disruptive is the key word for my next point. If either Sunogel or PolarityTE prove to provide complete regeneration with their products this will have major ramifications for someone like Dr. Spiegel. Suddenly, his main calling card and source of business (i.e. the ability to perform surgeries with minimal or mostly hidden scars) is rendered mostly moot.
Look, I'm not saying that he's making this claim callously. Most likely he's just ignorant to all the research being done because he doesn't have the time to dig into it like a lot of us on this site do or is being cautious about specific timelines. However, keep in mind that products like Sunogel and SkinTE threaten his business. Think about it. Let's say he somehow knew for a fact that a product was coming that provided complete regeneration for wounds. Do you think he'd gush about it? Absolutely not, it would cost him business in the interim because people would just want to wait for the product and then go to the least expensive surgeon (that was still trustworthy and skilled) they could find.
Regenerative medicine is incredibly disruptive and as such there is going to be a lot of misinformation as it picks up steam (the FDA streamlining the process should rapidly accelerate this). This will come from both sides; doctors, surgeons and manufacturers of lesser products feeling threatened by the sea change and "unscrupulous actors" (as the FDA puts it) that seek to use regenerative medicine as a buzz term to get people to fork over tons of money for ineffective treatments.
Nothing about PolarityTE or Sunogel suggests they are the latter to this point. Both are even going through clinical studies even though they don't have to. That's a great sign because it shows they aren't just going with the path of least resistance to make a buck.
I think the disruptive part is something many people don't realize about these emerging technologies. Going from the current scar revision processes to scarless healing is just like going from the basic brick phone to an iPhone -->even proving that scarless healing is possible will change EVERYTHING --- the scar market will be a small drop in the ocean. Think of the skincare markets, the aging treatment markets, the hair loss markets, even plastic surgery will be affected. Plus companies will start shifting technology towards refining scarless technology rather than stick with what they have.So IF (big IF) this technology proves to work, things will speed up at an exponential pace.
1 hour ago, Raster said:I think the disruptive part is something many people don't realize about these emerging technologies. Going from the current scar revision processes to scarless healing is just like going from the basic brick phone to an iPhone -->even proving that scarless healing is possible will change EVERYTHING --- the scar market will be a small drop in the ocean. Think of the skincare markets, the aging treatment markets, the hair loss markets, even plastic surgery will be affected. Plus companies will start shifting technology towards refining scarless technology rather than stick with what they have.So IF (big IF) this technology proves to work, things will speed up at an exponential pace.
The real crux of the matter is whether it will be affordable for those who really need the damn thing.
 Acne.org Products
Acne.org Products