It Means That a Product Is Less Likely to Cause an Allergic Reaction. However, Consumers Should Know That Any Cosmetic Product Can Cause Allergic Reactions, Even Those Advertised as Hypoallergenic.

The Essential Info
Hypoallergenic is a term that means “reduced allergy.”
However, the U.S. Food and Drug Administration (FDA) does not require the manufacturers of skincare products to prove hypoallergenic claims with any testing, and there are no scientific studies showing that hypoallergenic skincare products lead to fewer allergic reactions than other products sold for the same purposes. In other words, just because a product says it is hypoallergenic does not mean it is less likely to cause a reaction.
However, common sense tells us that companies that claim a product is hypoallergenic are at the very least taking into consideration the potential for their ingredients to cause allergy.
If you experience irritation or other adverse reactions to a product, a doctor may be able to help you identify the ingredient that caused the allergy by performing a patch test. Choosing products that do not contain that ingredient is the best way to avoid allergic reactions.

The Science
- Signs of an Allergic Reaction
- Identifying the Ingredient(s) That Cause Allergies
- How Often Do Allergic Reactions Occur?
- Product Safety Testing
- Conclusion
You have probably seen the claim “hypoallergenic” advertised with various skincare products, including those used to treat acne.
According to the FDA, products labeled as hypoallergenic are “products that manufacturers claim produce fewer allergic reactions than other cosmetic products.”1

Many people with sensitive skin might expect hypoallergenic products to be certain not to irritate their skin. But this may not be the case because hypoallergenic claims are not necessarily supported by any testing, standards, or regulations.

“Consumers concerned about allergic reactions from cosmetics should understand one basic fact: there is no such thing as a ‘nonallergenic’ cosmetic, that is, a cosmetic that can be guaranteed never to produce an allergic reaction.”1
There are three main reasons that hypoallergenic products may still cause reactions:
1. Lack of Regulation
First, the term hypoallergenic itself is not regulated. Some manufacturers may define it differently than others, and there is no guarantee that hypoallergenic products are any safer for people with sensitive skin. As stated by the FDA:

“There are no Federal standards or definitions that govern the use of the term ‘hypoallergenic.’ The term means whatever a particular company wants it to mean. Manufacturers of cosmetics labeled as hypoallergenic are not required to submit substantiation of their hypoallergenicity claims to FDA.”1
Since 1974, the FDA has sought to prohibit manufacturers from making hypoallergenic claims about their products unless scientific studies backed up those claims. But their efforts were legally blocked by the U.S. Court of Appeals for the District of Columbia. This may change at some point in the future if the FDA decides to pursue it. However, the FDA has been successful in requiring products marketed in the U.S. to carry a list of ingredients. This enables consumers to avoid products that contain substances to which they may be allergic. Checking the ingredients list on products is the best way to avoid allergies if you know that you are allergic to a particular ingredient.1,2
2. People Are Genetically Different
We are all genetically different, so our skin is different too. What causes an allergy in one person may not cause an allergy in another.

3. Products Contain Many Ingredients
A product can contain dozens of ingredients. While ingredients that are known to commonly cause allergic reactions are no longer used in cosmetics in most countries, there is a chance that a small percentage of the population may still experience allergic reactions to some ingredients, particularly those that have been recently introduced into products. Consequently, there is no way to be sure that at least some people will not experience an allergy to some products.1,2
Signs of an Allergic Reaction
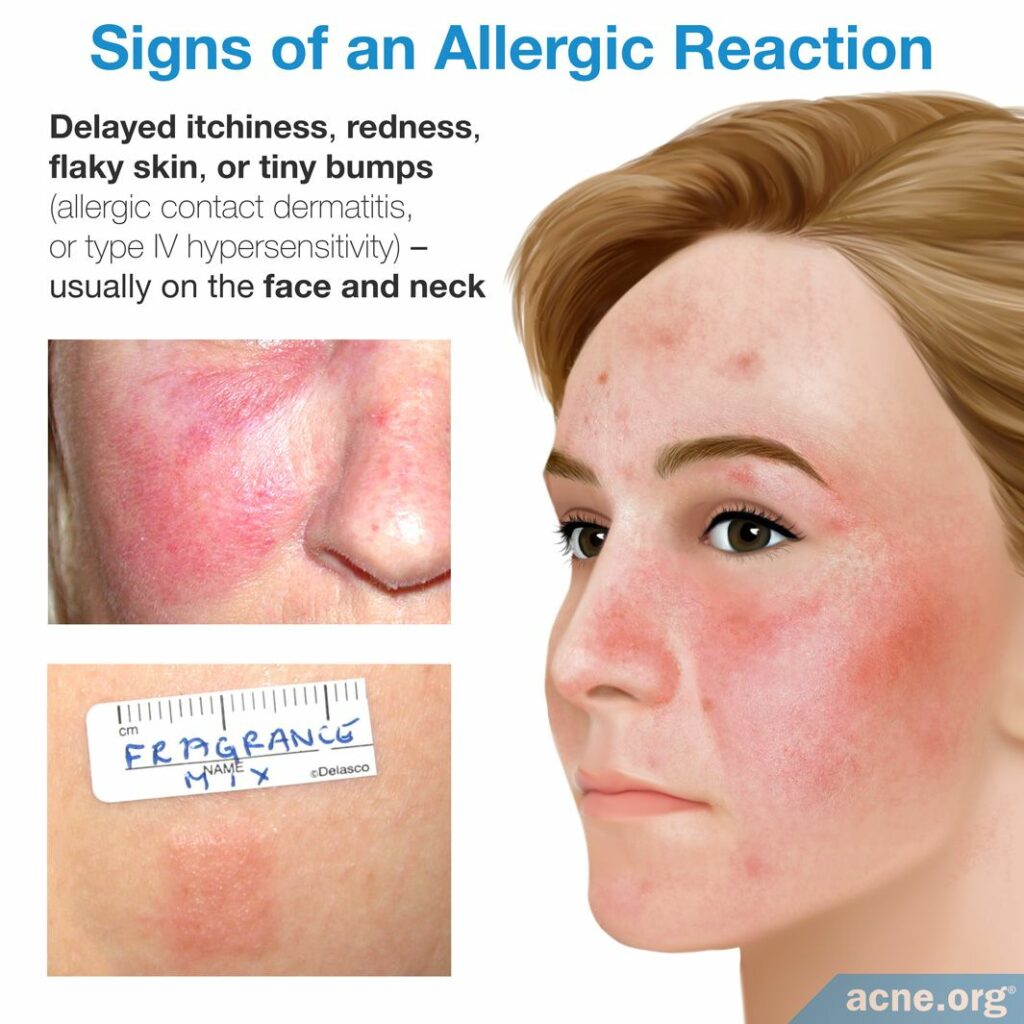
Irritations and other possible allergic reactions that you should be aware of include the following:
- Most common with skincare products: Delayed itchiness, redness, flaky skin, or tiny bumps (allergic contact dermatitis, or type IV hypersensitivity) – usually on the face and neck
- Immediate itching, burning, or stinging (irritant contact dermatitis)
- Redness and skin irritation (contact urticaria)
- Bumps on the skin (folliculitis)
- Sudden onset of acne-like lesions on the skin (acneiform eruptions)
- Sensitivity to the sun (photosensitization, photoirritation, or phototoxic dermatitis)
- Dark marks (hyperpigmentation, hypopigmentation, and other pigmentary disorders)
- Worsening of a previously established skin disorder2-4
Identifying the Ingredient(s) That Cause Allergies
If a person has an allergic reaction to a skincare product and seeks medical attention, doctors can use a standard test to figure out exactly which ingredient in the product is the irritant.
- Skin prick test: Also known as a scratch test, this test helps doctors detect immediate allergic reactions. When performing a skin prick test, a doctor gently pricks the skin – normally the forearm – with a set of known allergens and then evaluates how the skin reacts to each of those allergens after waiting for a certain period of time. Doctors also take into consideration the patient’s clinical history. Doctors typically use the skin prick test to check for food allergies and environmental allergies like those to pollen and pet hair, but it can also detect reactions to ingredients in skincare products.
- Patch testing: This test can detect delayed allergic reactions and is generally used for diagnosis of contact dermatitis.1,3,5 When performing a patch test, a doctor applies a sticky patch treated with a set of known allergens to the patient’s skin, typically on the patient’s back. After wearing the patch for 48 hours, the patient returns to the doctor’s office, where the doctor removes the patch and looks for signs of an allergic reaction on the skin.3 The FDA-approved patch test kit that most doctors in the U.S. use is called the thin-layer rapid-use epicutaneous – or TRUE – test. It now includes 35 different allergens that patients can be tested for, as selected by the North American Contact Dermatitis Group and European Community Cosmetics Directive.5 Fragrances and preservatives are the most common ingredients in cosmetics that people are allergic to. However, virtually any type of skincare ingredient may cause a skin reaction in some people.3
What to Do after You Have Identified Your Allergens
Once your doctor has performed the skin prick or patch test, she can advise you on what skincare ingredients you need to avoid. Before purchasing any skincare product, check the ingredient list to ensure the ingredient you are allergic to is not included. Do not rely on the “hypoallergenic” label, since even products marked hypoallergenic may still contain ingredients that you personally may be allergic to.
To help people find products that will not cause an allergic reaction, the American Contact Dermatitis Society has created an app called the Contact Allergen Management Program (CAMP). The app allows the user to input ingredients that trigger an allergic reaction in him or her and then generates a list of products free from these ingredients.6
How Often Do Allergic Reactions Occur?
Irritations that fall under the category of allergic contact dermatitis (ACD) are most likely to happen with skincare products. Suspected allergic contact dermatitis to makeup or skincare products is also the most frequent reason for people to go to their dermatologist for patch testing.6 Fortunately, adverse reactions such as these are not common–regardless of whether a product is labeled “hypoallergenic” or not–because new ingredients usually undergo extensive safety testing before hitting the market.4 According to a 2013 report in the scientific journal Dermatitis:

“Modern cosmetics are safe for most users, and adverse reactions are very rare because the manufacturers invest heavily in safety, quality control, and product testing before releasing the product to the market.”5 But despite safety testing, allergic reactions do happen. Several studies have tried to estimate how often they occur based on patch testing results.7-10 According to these estimates, somewhere between 9.8 and 23% of cosmetics users may experience an allergic reaction.8,9
Expand to read details of studies

A 2004 study in the American Journal of Clinical Dermatology found that 23% of women and 13.8% of men had an adverse reaction to a personal care product over a one-year period.8

A 2011 report in the journal Skin Therapy Letter compiled a set of seven studies that included 30,207 participants from the U.S. and the U.K. and found that 9.8% of the people studied tested positive for a skin allergy.9

When it comes to one particular type of skincare product–sunscreens–a 2018 article published in the journal Clinical Reviews in Allergy & Immunology reported that allergic contact dermatitis to sunscreens is very rare, occurring in less than 1% of users. When allergic reactions to sunscreen do occur, the culprit is usually an ingredient called benzophenone-3, also known as oxybenzone, which can also be a component of other personal care products.11
As noted, there is a variety of allergic reactions that can occur, and some are more severe than others. It can be difficult for researchers to estimate how often the less severe reactions occur. For instance, authors of various studies report ACD, or type IV hypersensitivity, occurs in less than 1% of the population.12-16

But according to a 2014 study in the journal Dermatologic Clinics, this estimate is probably low because not all patients who experience a skin allergy seek medical attention. Many simply stop using the product, and never report the allergy.4
Product Safety Testing
The process for testing the safety of new skincare products usually begins with testing on animals. After individual ingredients are tested on animals and appear to be safe, the final product is then sometimes tested on humans in early trials. Many companies skip this testing process because it is expensive and can take a long time to complete. Manufacturers can advertise their products as hypoallergenic, regardless of how much product testing they perform, and results from animals or small groups of people cannot represent how all people will respond. Also, if a new ingredient is causing allergies, it may be sold in products for quite some time before dermatologists can identify it as part of a trend.2,5
Conclusion
Any manufacturer can claim a product is hypoallergenic. Since all skincare products must include an ingredients list, consumers should refer to this list and avoid products that contain ingredients that they are allergic to.
References
- “Hypoallergenic” Cosmetics https://www.fda.gov/cosmetics/labeling/claims/ucm2005203.htm
- Draelos, Z. D. & Rietschel, R. L. Hypoallergenicity and the dermatologist’s perception. J. Am. Acad. Dermatol. 35, 248 – 251 (1996). https://www.sciencedirect.com/science/article/abs/pii/S0190962296903424
- Goossens, A. Contact-allergic reactions to cosmetics. J. Allergy (Cairo) 2011, 467071 (2011). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3065000/
- Park, M. E. & Zippin, J. H. Allergic contact dermatitis to cosmetics. Dermatol. Clin. 32, 1 – 11 (2014). https://www.ncbi.nlm.nih.gov/pubmed/24267417
- Alani, J. I., Davis, M. D. & Yiannias, J. A. Allergy to cosmetics: a literature review. Dermatitis 24, 283 – 290 (2013). https://www.ncbi.nlm.nih.gov/pubmed/24201464
- Zirwas, M. J. Contact dermatitis to cosmetics. Clin. Rev. Allergy Immunol. 56, 119‐128 (2019). https://www.ncbi.nlm.nih.gov/pubmed/30421329
- Tammela, M. et al. Patch testing with own cosmetics–a prospective study of testing and reporting of adverse effects to the Swedish Medical Products Agency. Contact Dermatitis 67, 42 – 46 (2012). https://www.ncbi.nlm.nih.gov/pubmed/22443095
- Orton, D. I. & Wilkinson, J. D. Cosmetic allergy, incidence, diagnosis, and management. Am. J. Clin. Dermatol. 5, 327 – 337 (2004). https://www.ncbi.nlm.nih.gov/pubmed/15554734
- Hamilton, T. & de Gannes, G. C. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther. Lett. 16, 1 – 4 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21611680
- Nielsen, N. H. et al. Allergic contact sensitization in an adult Danish population: two cross sectional surveys eight years apart (the Copenhagen Allergy Study). Acta Derm. Venereol. 81, 31 – 34 (2001). https://www.semanticscholar.org/paper/Allergic-contact-sensitization-in-an-adult-Danish-Nielsen-Linneberg/3aadbd2604376f37f16d97295d7765ee84f24bb0
- Nguyen, H. L. & Yiannias, J. A. Contact dermatitis to medications and skin products. Clin. Rev. Allergy Immunol. 56, 41-59 (2019). https://pubmed.ncbi.nlm.nih.gov/30145645/
- Eiermann, H. J. et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J. Am. Acad. Dermatol. 6, 909 – 917 (1983). https://www.ncbi.nlm.nih.gov/pubmed/7096650
- Romaguera, C. et al. Patch tests with allergens related to cosmetics. Contact Dermatitis 9, 167 – 168 (1983). https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0536.1983.tb04346.x
- Adams, R.. M. & Maibach, H. I. A five-year study of cosmetic reactions. J. Am. Acad. Dermatol. 13, 1062 – 1069 (1985). https://www.ncbi.nlm.nih.gov/pubmed/4078100
- de Groot, A. C. Contact allergy to cosmetics: causative ingredients. Contact Dermatitis 17, 26 – 34 (1987). https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0536.1987.tb02640.x
- Nielsen, N. H. & Menne, T. Allergic contact sensitization in an unselected Danish population. The Glostrup Allergy Study, Denmark. Acta Derm. Venereol. 72, 456 – 460 (1992). https://www.ncbi.nlm.nih.gov/pubmed/1362844
 Acne.org Products
Acne.org Products