Lactic Acid and Lactate Are Two Chemically Similar Molecules Created by Means of Chemical Synthesis, Catalyzing the Degradation of Sugars, Microbial Fermentation, and the Natural Synthesis Pathway

The Essential Info
Lactic acid and lactate are two forms of the same molecule that are used in thousands of skincare products.
The difference between the two comes down to the acidity of the product:
Lactic acid: In acidic products. Acts to exfoliate + moisturize.
Lactate: In non-acidic products. Acts to moisturize only.
That’s the long and short of it. The article below will get into the science of their chemical structures and methods of synthesis, so it’s pretty geeky. Why is this article on acne.org? To be honest, I was just curious about the difference between lactic acid and lactate and got a little carried away. If you’re more interested in lactic acid as a skincare ingredient, check out this article on lactic acid specifically.

The Science
Many skincare products on the market, including those labeled for acne-prone skin, contain lactic acid or lactate. While few studies have looked at lactic acid and/or lactate as an acne treatment, preliminary research suggests that these substances may potentially help with acne.1
Lactic acid is an alpha-hydroxy acid (AHA), which means it exfoliates the skin. Lactate is a component of the skin’s natural moisturizing factor (NMF), a mixture of substances found on the skin surface which keeps our skin hydrated and protected.2,3
The following is a scientific article describing the difference between lactic acid and lactate–two forms of the same molecule. If you’re feeling nerdy, have a read. But what it boils down to is that you’ll see both of these ingredients in skin care products depending on the pH (acidity) of the product:
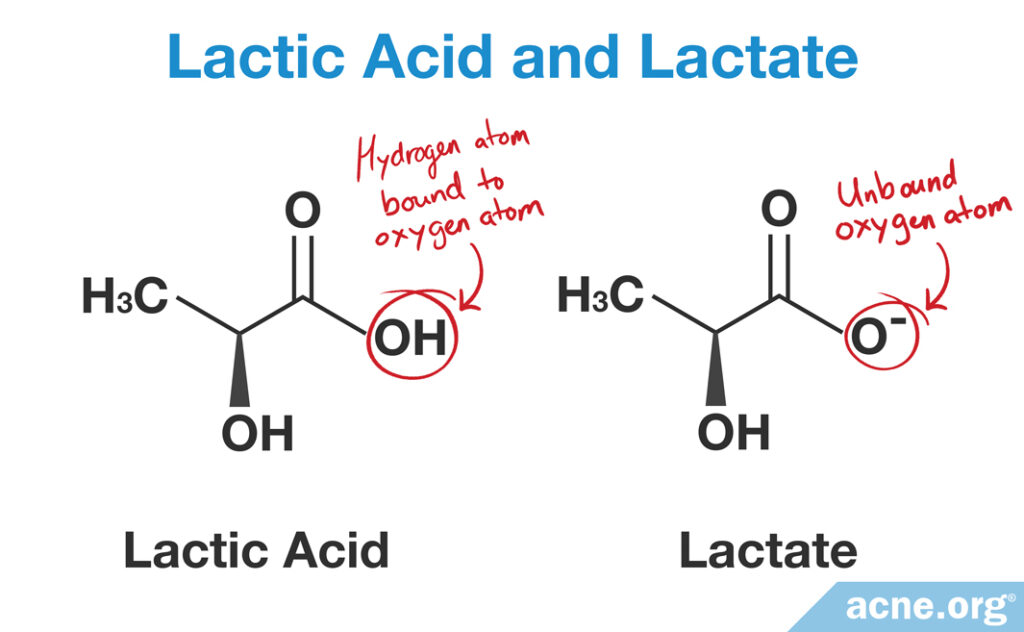
- If the product is an acidic (low pH) product, it will have lactic acid in it and will exfoliate the skin and moisturize the skin.
- If the product is alkaline/basic (high pH), it will have lactate in it and will moisturize the skin.
Chemical Structure of Lactate and Lactic Acid
When the pH in the surrounding environment is basic, we get lactate, whereas when the pH in the surrounding environment is acidic, we get lactic acid.
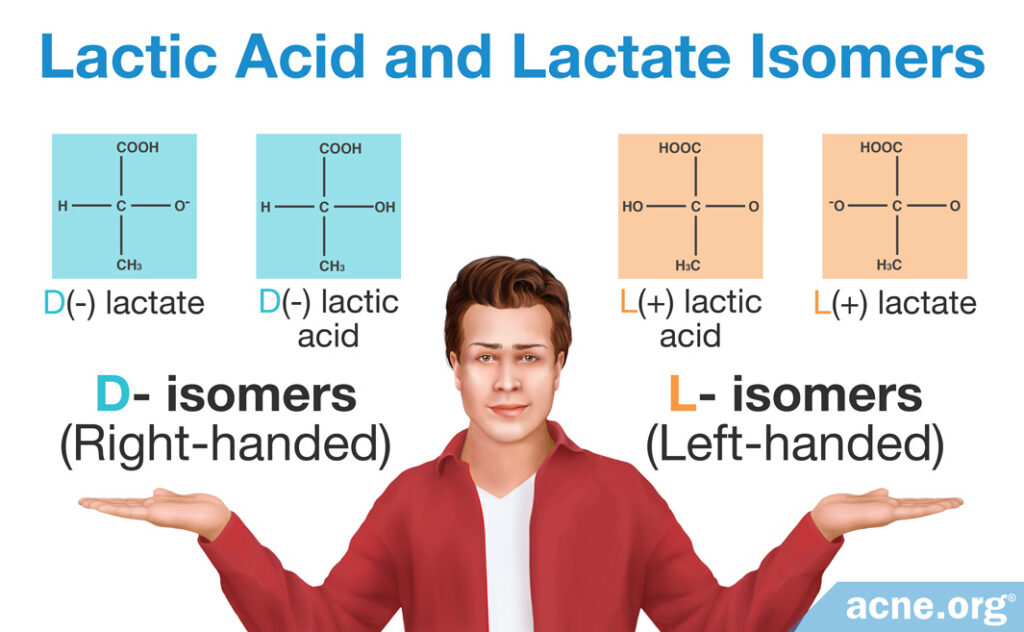
Depending on the orientation of atoms in the molecule, lactate and lactic acid come in D- or L- forms–“right-handed” and “left-handed,” respectively. This gives us four isomers (identical molecules with a mirror arrangement), with lactic acid and lactate coming in three forms:
- D-lactate, L-lactate (isomers)
- D-lactic acid, L-lactic acid (isomers)
- DL-lactic acid (discussed later in the article), DL-lactate
The D and L structures of lactate and of lactic acid are mirror images of each other. In other words, the D and L forms contain the same atoms, but the orientation of the atoms is mirrored.4-9
Manufacturers can synthesize lactic acid using a variety of processes, including chemical synthesis, catalyzing the degradation of sugars, and microbial fermentation.
Chemical synthesis
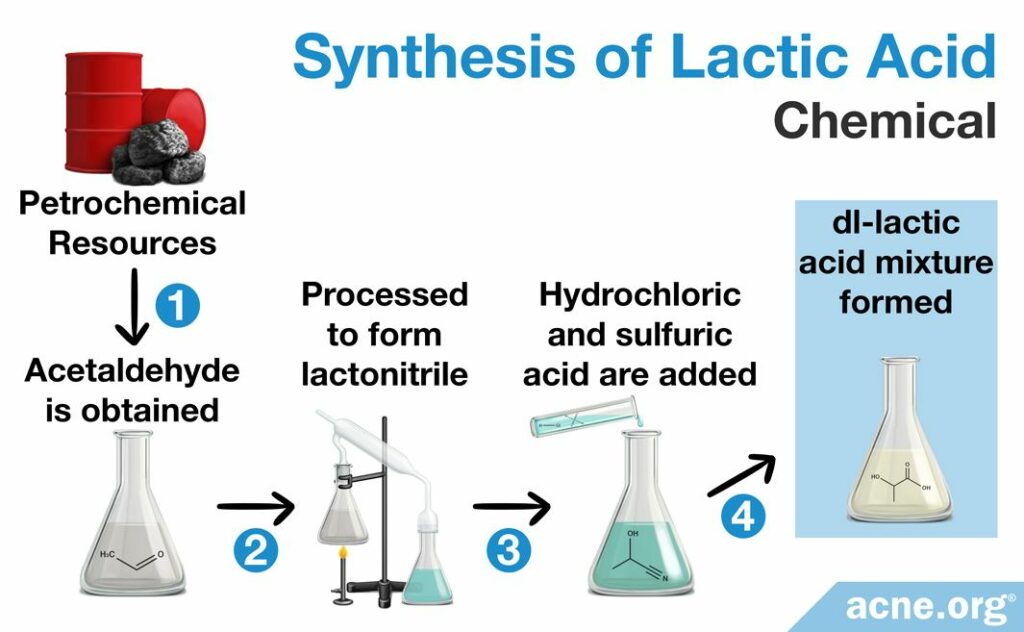
Scientists created the chemical synthesis pathway in the 1960s in order to satisfy the baking industry’s need for lactic acid. This process begins with chemical compounds made from oil, natural gas, or coal, called petrochemical resources. From these resources a special chemical known as acetaldehyde is obtained. Acetaldehyde then undergoes a variety of chemical processes to produce lactonitrile, which is purified* and, after the addition of either hydrochloric or sulfuric acid, hydrolyzed**, to produce lactic acid. The final product is always a mixture of the D and L forms, which is called a racemic DL-lactic acid mixture. This DL- mixture is useful if the producer desired both forms, but ineffectual if only one form was needed.4,10
*Here, purification is by distillation (a process of separating the component substances from a liquid mixture by selective evaporation and condensation).10
**Hydrolysis is the process by which a substance is broken down by chemical reaction with water.
Catalyzing the degradation of sugars
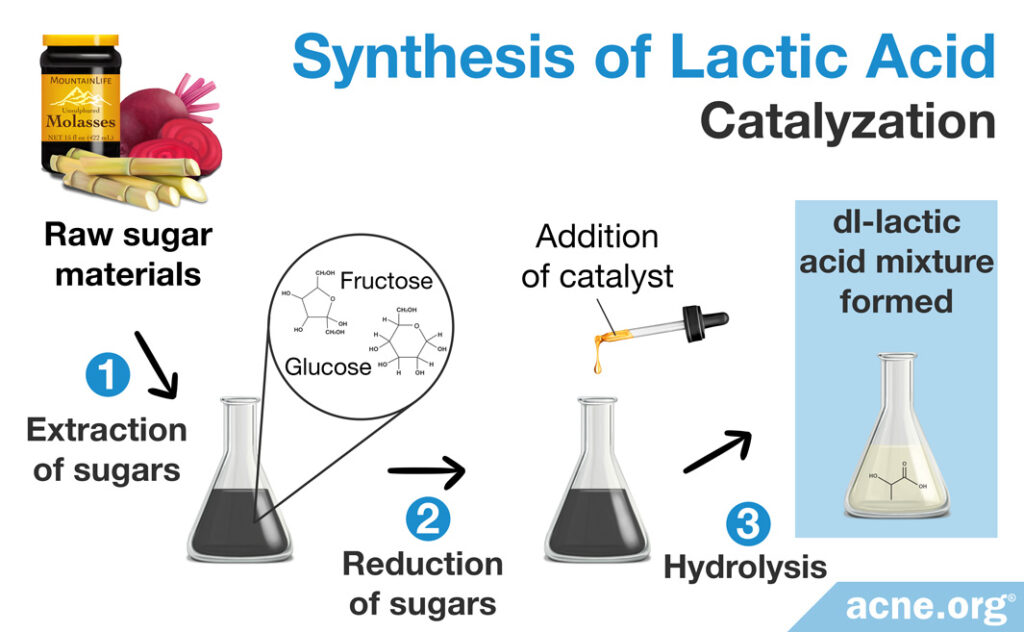
Manufacturers can make a racemic DL-lactic acid mixture also through catalyzing the degradation of sugars. After a meal, the body breaks down sugars and can use that energy to produce lactic acid. Scientists can simulate this process in a laboratory by catalyzing (speeding up the breaking down of) sugars to create lactic acid in a controlled environment. This process begins with raw sugar materials, such as sugarcane molasses, beetroot molasses, or a sugar in plants known as inulin, which contain high levels of glucose or fructose (simple sugars). Scientists then incorporate a catalyst which can break down these sugar molecules via hydrolysis. After the chemical reactions are complete, a racemic DL-lactic acid mixture is formed.11
Microbial fermentation
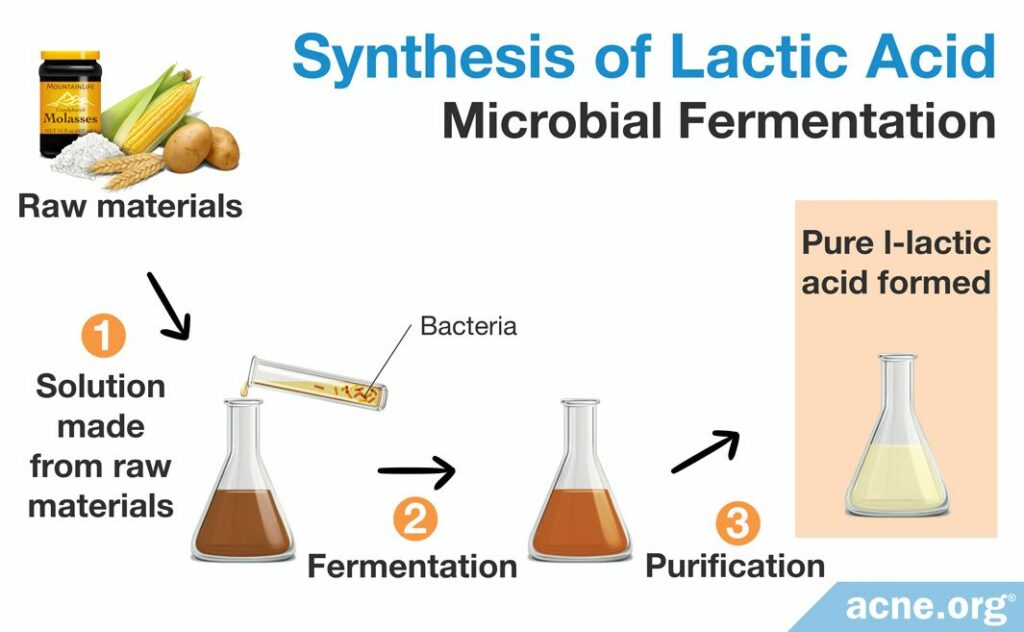
When a racemic DL-lactic acid mixture is not ideal, manufacturers can employ microbial fermentation to produce a pure L-lactic acid solution. Microbial fermentation is a process that takes advantage of microorganisms including bacteria and fungus that naturally break down sugars into easily usable chemicals. This process begins with raw biological materials like molasses, rye, corn, whey, or potato, which are made into a broth-like solution and incubated with a specific group of microorganisms that break down the starting raw material. After the microorganisms break down the sugars in a process known as fermentation, scientists take the broth, filter it, and treat it with sulfuric acid to produce an impure solution of lactic acid. They then purify this solution to obtain pure lactic acid. Scientists prefer microbial fermentation because it is able to produce only the L-lactic acid form instead of the racemic DL-lactic acid mixture formed by chemical synthesis or by catalyzing the degradation of sugars, which must be further purified to achieve a pure L-lactic acid solution. As scientists have not yet created a method for isolating the D-lactic acid form, producers prefer to create and use the L-lactic acid form.4,10,12,13
Natural Synthesis
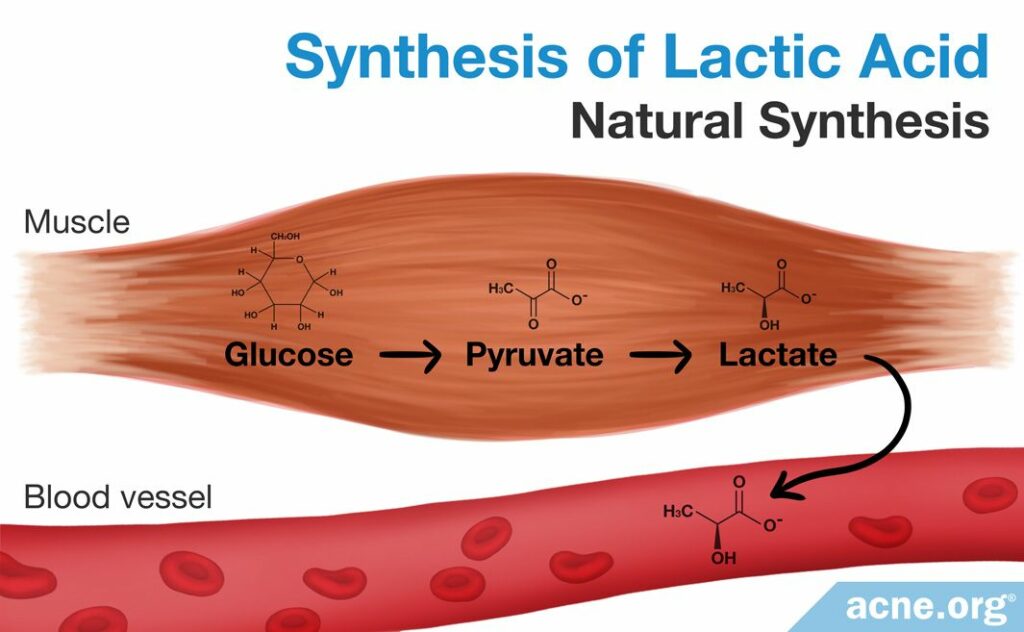
The human body can also naturally synthesize lactate and lactic acid. Lactic acid fermentation is a natural process that humans and other organisms employ to break down food molecules for energy in the absence of oxygen. This type of energy production occurs mainly in muscle cells during periods of intense exercise. A process known as the “lactic acid cycle” takes a product of glucose degradation called pyruvate and converts it into lactate through a number of chemical steps. This method, although not used in laboratory synthesis of lactate or lactic acid for commercial purposes, is a natural pathway through which lactate is created.8,14,15
Conclusion
Lactic acid and lactate are two chemically similar molecules created through chemical synthesis, catalyzing the degradation of sugars, microbial fermentation, and the natural synthesis pathway. Scientists prefer the microbial fermentation pathway because it produces a pure solution of the L-lactic acid form used in many commercial products, including skincare products that hydrate the skin. Lactic acid and lactate production is important for many industries, and understanding the four synthesis pathways provides an interesting view into how lactic acid and lactate are made.
References
- Garg, T., Ramam, M., Pasricha, J. S. & Verma, K. K. Long term topical application of lactic acid/lactate lotion as a preventive treatment for acne vulgaris. Indian J Dermatol Venereol Leprol 68, 137‐139 (2002). https://www.ncbi.nlm.nih.gov/pubmed/17656910
- Tang, S. C. & Yang, J. H. Dual effects of alpha-hydroxy acids on the skin. Molecules 23, 863 (2018). https://www.ncbi.nlm.nih.gov/pubmed/29642579
- Sugawara, T., Kikuchi, K., Tagami, H., Aiba, S. & Sakai, S. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci 66, 154‐159 (2012). https://www.ncbi.nlm.nih.gov/pubmed/22464763
- Wee, Y. J., Kim, J. N. & Ryu, H. W. Biotechnological Production of Lactic Acid and Its Recent Applications. Food Technol Biotechnol 44, 163 – 172 (2006). https://www.researchgate.net/publication/228357901_Biotechnological_production_of_lactic_acid_and_its_recent_applications_Food_Technol
- Fowler, J. Understanding the role of natural moisturizing factor in skin hydration. Pract Dermatol 36 – 40 (2012). https://practicaldermatology.com/articles/2012-jul/understanding-the-role-of-natural-moisturizing-factor-in-skin-hydration
- Kraft, J. N. & Lynde, C. W. Moisturizers: what they are and a practical approach to product selection. Skin Ther Lett 10, 1 – 8 (2005). https://www.ncbi.nlm.nih.gov/pubmed/15986082
- Illustrated Glossary of Organic Chemistry – Lactic acid, http://www.chem.ucla.edu/~harding/IGOC/L/lactic_acid.html
- Lactic acid, https://en.wikipedia.org/wiki/Lactic_acid
- Lactic Acid and Lactate, http://www.lactic-acid.com/lactate_and_lactic_acid.html
- Narayanan, N., Roychoudhury, P. K. & Srivastava, A. L(+) lactic acid fermentation and its product polymerization. Electronic J Biotechnol 7, 167 – 179 (2004). http://www.ejbiotechnology.info/index.php/ejbiotechnology/article/view/v7n2-7/520
- Cantero, D. A. et al. Selective transformation of fructose and high fructose content biomass into lactic acid in supercritical water. Cataly Today 225, 80 – 86 (2015). https://www.semanticscholar.org/paper/Selective-transformation-of-fructose-and-high-into-Cantero-Vaquerizo/1910ec4a54e0536950fc7fe4775f1fc2f2b435fb
- Zhou, S. et al. Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69, 399 – 407 (2003). https://www.ncbi.nlm.nih.gov/pubmed/12514021
- Poudel, P., Tashiro, Y. & Sakai, K. New application of Bacillus strains for optically pure L-lactic acid production: general overview and future prospects. Biosci Biotechnol Biochem 80, 642-654 (2016). https://pubmed.ncbi.nlm.nih.gov/26565947/
- Cori cycle, https://en.wikipedia.org/wiki/Cori_cycle
- Lactic acid fermentation, https://en.wikipedia.org/wiki/Lactic_acid_fermentation
 Acne.org Products
Acne.org Products