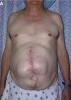
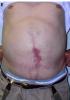
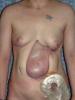
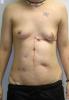
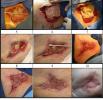
ecm no denatured: http://www.lifecell.com
the photos show wounds of operations dealt with a ECM no denatured (alloderm)
as they can see the treatment with ECM no denatured (acelullar) ,does not give perfect results, other elements are necessary in the puzzle, like tgf3, decorin, etc.
ecm no denatured: http://www.lifecell.com
the photos show wounds of operations dealt with a ECM no denatured (alloderm)
as they can see the treatment with ECM no denatured (acelullar) ,does not give perfect results, other elements are necessary in the puzzle, like tgf3, decorin, etc.
If it is non denatured you'd have absolutely no visible scar.
The intercellular cells and stem cells will be to create tissue through the fibrils, (if non denatured the fibrils/pores are not blocked up by excess collagen , hence no scar). BTW your never wounded, unwounded tissues are non denatured, they are constantly recreating site specific tissue.
If it is denatured you'd have scar. The fibrils would be blocked up with scar tissue, meaning the intercellular cells could not create site specific tissue through the fibrils.
ecm no denatured: http://www.lifecell.com
the photos show wounds of operations dealt with a ECM no denatured (alloderm)
as they can see the treatment with ECM no denatured (acelullar) ,does not give perfect results, other elements are necessary in the puzzle, like tgf3, decorin, etc.
If it is non denatured you'd have absolutely no visible scar.
The intercellular cells and stem cells will be to create tissue through the fibrils, (if non denatured the fibrils/pores are not blocked up by excess collagen , hence no scar). BTW your never wounded, unwounded tissues are non denatured, they are constantly recreating site specific tissue.
If it is denatured you'd have scar. The fibrils would be blocked up with scar tissue, meaning the intercellular cells could not create site specific tissue through the fibrils.
in the page of this ecm (alloderm) says that she is no denatured
perhaps this ecm no denatured, it needs decorin in 200nm as your you said once.
ecm no denatured: http://www.lifecell.com
the photos show wounds of operations dealt with a ECM no denatured (alloderm)
as they can see the treatment with ECM no denatured (acelullar) ,does not give perfect results, other elements are necessary in the puzzle, like tgf3, decorin, etc.
If it is non denatured you'd have absolutely no visible scar.
The intercellular cells and stem cells will be to create tissue through the fibrils, (if non denatured the fibrils/pores are not blocked up by excess collagen , hence no scar). BTW your never wounded, unwounded tissues are non denatured, they are constantly recreating site specific tissue.
If it is denatured you'd have scar. The fibrils would be blocked up with scar tissue, meaning the intercellular cells could not create site specific tissue through the fibrils.
in the page of this ecm (alloderm) says that she is no denatured
perhaps this ecm no denatured, it needs decorin in 200nm as your you said once.
IMO its Marketing... If you look at the picture there is scar in the tissue defect. My guess visually is that, lets say, 70% of the fibrils are blocked up (scarred) and 30% are non denatured (non scarred).
Therefor lets say, the wounds are 70% denatured (we cant confirm it.)
If it was 100% non-denatured you'd have normal tissue, exactly like the non scarred surrounding tissue.
However if they told you the product was denatured (i.e. the fibril require excess collagen, as a defence to a foreign body), well it doesn't sound right.
The fact is if it was 100% non denatured, the body would not regard it as a foriegn body it would completely accept it, it would resorb and it would not over express collagen.
kitoscell: inhibit tgf b1 like juvidex
http://www.acne.org/messageboard/KITOSCELL...EX-t261587.html
for my the best product than this on sale today
this is another product in phase 2: bloks collagen type 3
Fibrostat: http://journals.lww.com/plasreconsurg/Abst...ment_of.19.aspx
Home > January 1996 - Volume 97 - Issue 1 > Topical Putrescine (Fibrostat) in Treatment of Hypertrophic...
< Previous Abstract | Next Abstract > Text sizing:A A A
Plastic & Reconstructive Surgery:
January 1996 - Volume 97 - Issue 1 - pp 117-123
Articles
Topical Putrescine (Fibrostat) in Treatment of Hypertrophic Scars: Phase II Study
Dolynchuk, Kenneth N. M.D., Ph.D.; Ziesmann, Manfred M.D.; Serletti, Joseph M. M.D.
AbstractPrevious studies indicated that tissue transglutaminase plays a role in the cross-linking of type III procollagen in wound matrices and that this may be inhibited by 50 mM putrescine in vitro. For this reason, the clinical effect of 50 mM putrescine in a eutectic vehicle(Fibrostat) was studied in this phase II double-blind crossover study in 43 patients. Twenty of the patients had had recent surgery and were studied for product safety rather than efficacy. No toxic effects were observed in this group of patients, and only 1 of the 23 unoperated patients had a rash during treatment. The observed effect of Fibrostat versus sham treatment of 1 month's duration in active hypertrophic scar was a significant improvement of hypertrophy in 23 patients during the Fibrostat treatment arm, regardless of the order in which treatment was received. It is suggested that Fibrostat is a safe therapeutic agent for treatment of hypertrophic scar. Clinical examples to illustrate its use are given.
ecm no denatured: http://www.lifecell.com
the photos show wounds of operations dealt with a ECM no denatured (alloderm)
as they can see the treatment with ECM no denatured (acelullar) ,does not give perfect results, other elements are necessary in the puzzle, like tgf3, decorin, etc.
If it is non denatured you'd have absolutely no visible scar.
The intercellular cells and stem cells will be to create tissue through the fibrils, (if non denatured the fibrils/pores are not blocked up by excess collagen , hence no scar). BTW your never wounded, unwounded tissues are non denatured, they are constantly recreating site specific tissue.
If it is denatured you'd have scar. The fibrils would be blocked up with scar tissue, meaning the intercellular cells could not create site specific tissue through the fibrils.
in the page of this ecm (alloderm) says that she is no denatured
perhaps this ecm no denatured, it needs decorin in 200nm as your you said once.
IMO its Marketing... If you look at the picture there is scar in the tissue defect. My guess visually is that, lets say, 70% of the fibrils are blocked up (scarred) and 30% are non denatured (non scarred).
Therefor lets say, the wounds are 70% denatured (we cant confirm it.)
If it was 100% non-denatured you'd have normal tissue, exactly like the non scarred surrounding tissue.
However if they told you the product was denatured (i.e. the fibril require excess collagen, as a defence to a foreign body), well it doesn't sound right.
The fact is if it was 100% non denatured, the body would not regard it as a foriegn body it would completely accept it, it would resorb and it would not over express collagen.
perhaps you are right, and there is no ECM no denatured 100 percent on sale to today.
It reports annual regenerative medicine of the Armed Forces of the USA
http://www.afirm.mil/assets/docs/annual_report.pdf
hopefully this people soon sell a solution for the scars, see that they work much and seriously
@eternal:
Look at the top of the page. I have already mentioned it.*g*
http://www.redorbit.com/news/health/180465...skin/index.html
Here is something new for skin scarring. I hope that more drugs for scarring will be on the way. When I look back the last years had been very interesting. New lasers, new therapies. I am thrilled what the next years will bring us.
NEO
@eternal:
Look at the top of the page. I have already mentioned it.*g*
http://www.redorbit.com/news/health/180465...skin/index.html
Here is something new for skin scarring. I hope that more drugs for scarring will be on the way. When I look back the last years had been very interesting. New lasers, new therapies. I am thrilled what the next years will bring us.
NEO
Excellent post
you have some idea of when it can be on sale this drug?
hm. There is a homepage of the company, But I did not find anything concerning releas of the drug. I think it will take time, because a phase 3 study will follow. But it is the first time, that antisense drugs were used for fibrosis.
fingers crossed. next year.
NEO
http://www.biomediclabs.com/scar_tissue?gc...CFQseZwodrzEtSw
something new against fibrosis. maybe it works on skin.
Ok, we comment to people a little on links that put NEO.
EXC 001 : a antifibrotic drug, of the type kitoscell, I hope that it is far better. in phase 2.
I send an email to the company asking when to be on sale, if they respond will comment here.
the description of the drug draws attention powerfully to me (to be newness for my is something really good):
technology used: http://excaliard.com/technology.html
Technology
Excaliard has licensed a scarring and fibrosis program from Isis Pharmaceuticals, composed of:
1.A series of highly evolved 2a-O-methoxyethyl (2aMOE) modified ASO inhibitors of key regulators of fibrosis. The compounds are highly selective inhibitors of genes known to be regulators of pathological scar formation and fibrosis. In a variety of models, Excaliard's drugs have demonstrated significant and reproducible reductions in scar formation.
2.Broad, key intellectual property covering not only the specific ASOs, but also the basic technology, chemistry and manufacturing.
3.Access to Isis Pharmaceuticals industry leading technical expertise in oligonucleotide discovery and informatics, pharmacology and toxicology.
wow, this sounds impressive, makes see all other small including juvista. I hope that it is not another deception
about clinical trial: http://excaliard.com/news.html
And the other NEO link speacks of :
Serracor-NK :tablets available on sale, of low cost. dedicated rather to the natural treatment of the heart I create I am not myself clear, with benefit in fibrosis and scars, here a detailed description but:
http://www.biomediclabs.com/inc/sdetail/22463 300 caps 150 us dllrs
*Reduce scar tissue buildup, fibrosis, arthritic conditions, pain, swelling, inflammation and hundreds of other ailments. *Promotes healthy circulation by reducing excessive fibrin levels and reducing blood viscosity.*Lowers C-Reactive Protein levels in blood.* Supports a healthy inflammatory response and the natural healing process.* Combines two enterically coated, fibrinolytic proteases, Peptizyme SPA (Serrapeptase) and NattoSEBA(Nattokinase) that specifically address fibrin levels in the body.*
Anti Fibrosis (Scar Tissue Removal)
Enzymes eat scar tissue and fibrosis. Fibrosis is scar tissue and most doctors learn in anatomy that it is fibrosis that eventually kills us all. As we age, which starts at 27, we have a diminishing of the body's output of enzymes. This is because we make a finite amount of enzymes in a lifetime and we use up a good deal of them by the time we reach our 40's Cystic Fibrosis patients who have virtually no enzyme production to speak of, even as children usually don't make it past their 20's before they die of the restriction and shrinkage in the lungs from the formation of fibrosis or scar tissue.
So our body begins to dole out our enzymes with an eyedropper instead of with a tablespoon. Result: the repair mechanism of the body goes off balance and has nothing to reduce the over abundance of fibrin it deposits in nearly everything from simple cuts, to the inside of our internal organs and blood vessels. It is then when most women begin to develop things like fibrocystic breast disease, uterine fibroids, adhesions, and endometriosis. We all grow arterial sclerotic (meaning scar tissue) plaque, and have fibrin begin to spider web its way inside of our internal organs, reducing their size and function over time. This is why as we age our wounds heal with thicker, less pliable, weaker and very visible scars.
If we replace the lost enzymes, we can control and reduce the amount of scar tissue and fibrosis our bodies have. As physicians in the US are now discovering, even old scar tissue can be "eaten away" from surgical wounds, pulmonary fibrosis, and kidney fibrosis even keloid years after their formation. Medical doctors in Europe and Asia have known this and used orally administered enzymes for such for over 40 years!
Reduce scar tissue buildup, fibrosis, adhesions, arthritic conditions, pain, swelling, inflammation and hundreds of other ailments. Serracor's formulation is more complete and essential than a daily vitamin. Replace the enzymes that Mother Nature has taken away from us as we age. Serracor-NK is radically more advanced than today's leading systemic enzyme blends. Serracor-NK contains several sources of anti-oxidant fighting ingredients, as well as the full spectrum of proteolytic (protein digesting) enzymes, enterically coated to adapt to a wide spectrum of PH levels. Serracor's exclusive formulation contains several highly concentrated digestive enzymes that enable the body to digest fats, proteins, sugars, and carbohydrates. These digestive enzymes convert the food we eat into energy rather than stored fat.
it is needed to investigate but
They continue raising all the news that find, good work boys.
Decorin at 200nm makes fibroblast formation static, enabling the fibrils to stay slender; scar free healing has been done.
In center of investigation of the armed forces of the USA, they are investigating thanks to God, with decorin. hopefully they find a solution good. surely they are going to on sale put a drug quickly and not like other laboratories like renovo that only test interminable to speculate on with bonds and action in stock-market of values.
not only with decorin, they are at the same time investigating with many things, I have much faith to him to the investigation in the armed forces of the USA.
Ok, now seems that juvista to sell its rights of sale to another company/signature in North America.
Renovo to sell juvista anywhere in the world exept North America, there under another company.
also to put 5 million to make another test of phase 3 in 2013.
this drug almost does not make difference and this people serve to make business with action.
hopefully they leave better things before it leaves east product on sale, that continue making interminable phases of test.
http://www.renovo.com/documents/renovo_205EC.pdf
audio: http://mediaserve.buchanan.uk.com/2010/ren...10/lrframes.htm
@eternal:
There is no problem with renovo. Shire has the rights to market juvista in USA,Canda,Mexico and Renovo has the rights to market juvista in Europe and the rest of the world.
Phase 3 trial in europe will report back 1 quarter 2011. So nothing wrong here. They only want to do another phase 3 trial. And it does do a big difference in scar tissue regeneration, but they have to use a particular dosage of juvista (500 microl).
BEcause of the worldwide crysis they did not pay any bonus to the managers and they changed the license agreement with Shire. Nothing to be afraid of.
NEO
@eternal:
There is no problem with renovo. Shire has the rights to market juvista in USA,Canda,Mexico and Renovo has the rights to market juvista in Europe and the rest of the world.
Phase 3 trial in europe will report back 1 quarter 2011. So nothing wrong here. They only want to do another phase 3 trial. And it does do a big difference in scar tissue regeneration, but they have to use a particular dosage of juvista (500 microl).
BEcause of the worldwide crysis they did not pay any bonus to the managers and they changed the license agreement with Shire. Nothing to be afraid of.
NEO
I do not see necessity of one second part of phase 3 in 2013, so that not to make parallel with which this being made now?
To test in 2013 implies that it is finished in 2015 and the drug to be on sale minimal just in 2016
The ideal to be after the first part of phase 3, leaves on sale, but I do not believe they do that it
It gives the sensation me of which they stretch the time for a drug that is very simple.
@eternal:
the second phase 3 trial is done because they had trouble with the phase 2 trial, maybe you remember this. i read about it. the first phase 2 trial did not meet it's significant endpoint. so they did it a second one. that's the reason why it took longer for juvista to be available.
in your own link you find the answers. the results of the first phase 3 trial will be used for Shire's FDA approval process. as you can see, they will not wait till 2015. they will use the results of the phase 3 trial to market juvista. the second phase 3 trial is at the moment not needed but they are doing ut because of their bad phase 2 trial in the past. and the second phase 3 trial will report back in 2013.
you will see it, next here we will have juvista. and in a recent interview prof. ferguson said juvista is the most advanced treatment for scar regeneration. so let's hope it works next year.
NEO
@eternal:
the second phase 3 trial is done because they had trouble with the phase 2 trial, maybe you remember this. i read about it. the first phase 2 trial did not meet it's significant endpoint. so they did it a second one. that's the reason why it took longer for juvista to be available.
in your own link you find the answers. the results of the first phase 3 trial will be used for Shire's FDA approval process. as you can see, they will not wait till 2015. they will use the results of the phase 3 trial to market juvista. the second phase 3 trial is at the moment not needed but they are doing ut because of their bad phase 2 trial in the past. and the second phase 3 trial will report back in 2013.
you will see it, next here we will have juvista. and in a recent interview prof. ferguson said juvista is the most advanced treatment for scar regeneration. so let's hope it works next year.
NEO
What I say is that to be on sale after finalized the second part of phase 3, that is for 2015
second part of phase 3 begins in 2013 and it finishes surely in 2015.
I know that in 2011 to leave a report with photos, that does not matter to me, matter to me when to be juvista in the drug stores to be able to buy.
By the way I hope that the results of revision of scar are not as mediocre as those that we saw in phase 2.
no, as i wrote above and as your link mentions shire uses the data from the phase 3 trial for fda approval in 2011 and then you can buy it. why shall they wait till 2013?
i still believe it will be available next year. shire cannot pay renovo money for doing nothing. so the product must be on the shelf in the next year. and i am really believing that if the results are very good they will skip the second phase 3 trial because they don't need it.
NEO
Bad news
Friday sends an email to renovo, today receipt answer
I asks to him when to leave juvista on sale and to respond this:
Dear ++++++,
Thank you for your email and interest in our products and clinical trials.
Unfortunately, as the clinical trial process and obtaining of the relevant licenses can take years, we donat yet know when Juvista will be available to purchase or get on prescription.
Iam sorry we are unable to help you further at this time.
Kind regards
Steph Burgess
Volunteer Recruitment Administrator
Renovo
Tel: 0161 276 7130
Fax: 0161 276 7230
Email: [email protected]
Website: www.renovo.com
@eternal:
What did you expect from an volunteer recruitment administrator?
It's not his job to know anything about the product. He belongs to the human resources crew.*g*
And as he said the trials are going on. ASk the FDA or other institutions why they need so much
time for the approval process.
As we all know we will have to wait at least one year. Because the phase 3 trial will then report back and then they will do a FDA approval and then we will see. Maybe at the end of next year. I don't know. With a little bit of luck we will get juvista sooner then later.
NEO
some paper links:
http://www.ncbi.nlm.nih.gov/pubmed/20223988
http://www.ncbi.nlm.nih.gov/pubmed/20218919
@eternal:
What did you expect from an volunteer recruitment administrator?
It's not his job to know anything about the product. He belongs to the human resources crew.*g*
And as he said the trials are going on. ASk the FDA or other institutions why they need so much
time for the approval process.
As we all know we will have to wait at least one year. Because the phase 3 trial will then report back and then they will do a FDA approval and then we will see. Maybe at the end of next year. I don't know. With a little bit of luck we will get juvista sooner then later.
NEO
some paper links:
hopefully you are right
I have investigated on the processes of FDA and sometimes phases 4 are made, hopefully this is not the case
antifibrotics drugs as kitoscell has approval of FDA, why renovo take's so long? i don't know
 Acne.org Products
Acne.org Products