16 hours ago, surgical scar said:I don't "think". I need to see they have or are making the device for scars. Just because you think they should it doesn't mean they will. They haven't even said that they are making a device for scars. Dumb assumption.
I don't claim to be following these companies anymore, as I have realized there is no point waiting around for them in vain for years. So, if you have seen something promising for scars you advocate, post it.
Cytrellis has created a system including a hand-held device and consumables which is designed to treat large surface areas on the face in a precise and user friendly manner. The device includes a skin-coring mechanism designed to precisely control location and depth of micro-excisions.
It says on their site. They couldnt control location if it was a one size fits all device. Its not that I think they should, its their doing a controlled clinical study, they couldnt state their title of micro coring scarsif they were also micro coring everywhere else eg had no control. Its just common sense if your used to reading science journals. They dont use words for the sake of it in academic writing.
I dont understand the negativity tbh. It is what it is,they dont claim to grow new skin, and its not hard to conceptualise how this might help. Think about morbid obese people, our skin can stretch a long way. Its just a very clever way to remove tissue without triggering the scar pathway wound healing response.
On Ž8Ž/Ž3Ž/Ž2019 at 11:45 AM, Tony82 said:
RXI-109 is not in active development. The company that was developing it, RXi Pharma, has changed its name to Phio Pharma and are focusing on oncology. They have been trying to sell their dermatology assets with no luck because of the poor results.
How do I know? I invested in that firm and lost 90% of my money.
Hey sorry to hear about the money. ![]()
23 minutes ago, AlexZ77 said:So how far are we from a treatment that will give us perfect scarless skin? 10 years away? Or more like 30 years way?
I'd put my money on5 years at the earliest and 15 years at the latest. Honestly, I don't have much hope in Sunogel for several reasons. They named their product Wizgel, have somewhat suspicious demostration photos, and don't seem to be getting any attention from the medical community in general. Perhaps these are superficial reasons, but it's my just my two cents. Advances in regenerative healing are inevitable, but unfortunately quite slow as a consequence of how new they are.
The more I think about this problem, the more I realize acne scarring probably can't truly be fixed, at least in the foreseeable future. Humans don't really understand how to regenerate skin yet, much less regenerate it to fit the curves and contractions of the face. We would have to be able to perfectly control the height and texture ofregenerated skin. You also have to consider that people often have very small pits that litter the entire surface of the skin. Are all of these hundreds of pits going to be excised and regenerated perfectly? Even if this is possible, it's hard to believe this would leave a smooth result, like the pre-scarred skin.
And what about areas of uneven texture that aren't necessarily pits, but shallow, jagged areas? Would the skin just be removed across a wide area?
I'm usually the biggest proponent of the wonder and magic of science, but this problem of existing facial scarring seems nearly insurmountable. Does anyone have any ideas or methods they've read about that seem realistic for treating this some day?
I really don't want to be a downer. I hate the people in forums that vent their emotions by squashing the hopes of others, but I feel utterly hopeless with regards to ever being able to treat this.
1 hour ago, PTAA said:The more I think about this problem, the more I realize acne scarring probably can't truly be fixed, at least in the foreseeable future. Humans don't really understand how to regenerate skin yet, much less regenerate it to fit the curves and contractions of the face.
You say "humans don't know how to perfectly regenerate skin" but the human body however does. People aim to enable to the body to "do its trick" which is something they either already know how to do or are close to being able to.
58 minutes ago, Lapis lazuli said:You say "humans don't know how to perfectly regenerate skin" but the human body however does. People aim to enable to the body to "do its trick" which is something they either already know how to do or are close to being able to.
I see what you're saying, and I really don't want to sound like a downer, but how do we know that the body knows how to regenerate from a scar. As far as I'm aware, the body can only regenerate from a wound, not scars, since scars don'tgenerate an immune response. We would have to excise all the layers of the scar first, creating a full thickness wound. Are there any cases of a largefull thickness wound healing scarlessly in humans? I'm genuinely curious and not trying to be confrontational.
1 hour ago, PTAA said:I see what you're saying, and I really don't want to sound like a downer, but how do we know that the body knows how to regenerate from a scar. As far as I'm aware, the body can only regenerate from a wound, not scars, since scars don'tgenerate an immune response. We would have to excise all the layers of the scar first, creating a full thickness wound. Are there any cases of a largefull thickness wound healing scarlessly in humans? I'm genuinely curious and not trying to be confrontational.
Yeah, that's the idea; that the body regenerates tissue after an injury instead of sealing off the wound as fast as possible through creating a scar which it does to avoid possible death due to infection.
Small wounds heal without scarring. Larger ones don't unless the body, I guess in a certain way is tricked into thinking there's no risk of infection? Not sure. I think there's something on Sunogel's site where they say something about the exact approach/idea/concept.
21 hours ago, PTAA said:The more I think about this problem, the more I realize acne scarring probably can't truly be fixed, at least in the foreseeable future. Humans don't really understand how to regenerate skin yet, much less regenerate it to fit the curves and contractions of the face. We would have to be able to perfectly control the height and texture ofregenerated skin. You also have to consider that people often have very small pits that litter the entire surface of the skin. Are all of these hundreds of pits going to be excised and regenerated perfectly? Even if this is possible, it's hard to believe this would leave a smooth result, like the pre-scarred skin.
And what about areas of uneven texture that aren't necessarily pits, but shallow, jagged areas? Would the skin just be removed across a wide area?
I'm usually the biggest proponent of the wonder and magic of science, but this problem of existing facial scarring seems nearly insurmountable. Does anyone have any ideas or methods they've read about that seem realistic for treating this some day?
I really don't want to be a downer. I hate the people in forums that vent their emotions by squashing the hopes of others, but I feel utterly hopeless with regards to ever being able to treat this.
to all these questions . your acne scars, uneven textures , jagged areas small pits whatever the answer is microcoring , market release end of 2019
cytrelis patent says:
Technologies, methods, and/or devices described herein can be used for treatment of scars. Scars are characterized by fibroblast proliferation and overexpression of collagen that crosslinks and aligns in one specific direction. Scar tissue is often inferior to healthy tissue, e.g., scars in the skin are less resistant to ultraviolet radiation, lack sweat glands and hair follicles, and are of inferior appearance. Scars can be caused by a variety of conditions, e.g., trauma, abrasion, acne etc., on a variety of tissues. Current treatment of scars include chemical peels, filler injections (e.g. collagen), dermabrasion, laser treatment, radiotherapy, dressing, and steroids, all of which can have significant limitations and/or side effects. Technologies, methods, and/or devices herein can be used to debulk scars by removing microcores from the scar, thus breaking up the hardened tissue and allowing healthy tissue to grow into the microcavities. For example, technologies, methods, and/or devices described herein can be applied to scars on skin or other tissue, such as muscles, e.g., scars on the heart muscle after myocardial infarction.
[155] Technologies, methods, and/or devices described here in can be used for removal of tattoos. During tattooing, skin is penetrated by a needle carrying ink, and ink particles are inserted into the dermis. After the healing process, the ink pigment remains trapped within fibroblasts, ultimately concentrating in a layer just below the dermis/epi dermis boundary, where it remains stable. Tattoo removal techniques involve using lasers to break up the pigments, upon which they are cleared by the body's immune system.
It can treat:
In certain embodiments, technologies, methods, and/or devices described herein can be useful for removal of, e.g., redundant or excess skin, pigment, hair follicles, and/or vessels in the skin, and/or for treating acne, allodynia, blemishes, ectopic dermatitis,
hyperpigmentation, hyperplasia (e.g., lentigo or keratosis), loss of translucency, loss of elasticity, melasma (e.g., epidermal, dermal, or mixed subtypes), photodamage, rashes (e.g., erythematous, macular, papular, and/or bullous conditions), psoriasis, rhytides (or wrinkles, e.g., lateral canthal lines ("crow's feet"), age-related rhytides, sun-related rhytides, or heredity -related rhytides), sallow color, scar contracture (e.g., relaxation of scar tissue), scarring (e.g., due to acne, surgery, or other trauma), skin aging, skin contraction (e.g., excessive tension in the skin), skin irritation/sensitivity, striae (or stretch marks), tattoo removal, vascular lesions (e.g., angioma, erythema, hemangioma, papule, port wine stain, rosacea, reticular vein, or telangiectasia), or any other unwanted skin irregularities.
It is so simple removing skin in a micro dimensions the skin doesnt get scarring the microholes close with healthy skin this is because of fibroblast proliferation and migration into the microcavities. the mechanical stress is so small that myofibroblasts ae not activated and then the skin doesnt contractdoenst get scars. the key is to remove 20-25% of the scar in every season so after 5seasons you will get scar removal. the 20-25% numberis not random scientists have shown that removingt 25% of the skin with microcoring doesnt lead to scar. nothing else in the market today can remove scars like that
17 hours ago, Lapis lazuli said:Yeah, that's the idea; that the body regenerates tissue after an injury instead of sealing off the wound as fast as possible through creating a scar which it does to avoid possible death due to infection.
Small wounds heal without scarring. Larger ones don't unless the body, I guess in a certain way is tricked into thinking there's no risk of infection? Not sure. I think there's something on Sunogel's site where they say something about the exact approach/idea/concept.
this is how microcoring works basically , you said it of your own small wounds doenst get scarring , microcoring remove scars in micro scale . the microcavities doesnt get scars... something you may dont know is that scars have fibroblasts , if you see a histological examination of a scar you can see fibroblasts there... this happen because myofibroblasts are not something different than fibroblasts they are the same cells but they have activated from inflammatory responds . so more inflammation means more myofibroblasts more scarring , very small inflammation means less myofibroblasts , more fibroblasts no scarring , after scar formation myofibrblasts get apoptosis or return back to previous situation , fibroblasts , thats why scars have fibroblasts so a microcavity is a microcavity it doenst matter where it is if near the microcavity has normal skin or ''scarring'' . the mechanism is the same .... fibroblast proliferation and migration into the microcavity. to finish this is howDexIEME (sunogel) works it blocks myofibroblasts by plasticity converting them into fat cells and this leads to fibroblasts proliferation and migration into the three-d cross linked hydrogel (DexIEME) which is biodigradable bythe human body
25 minutes ago, Sibel said:DamnBoy: It sounds so promising that it is hard to believe.. well the end of 2019 is close..
MD. Cytrelliss technology should be available by the end of2019. this is what they are saying, also a second company named recros medica plan to release their microcoring machine at the end of 2019 BUT because they are using 1,5mm diameter of core needles it has a danger for scarring so they are closing the microcavities with an elastic membrane and the skin is tightening, cytrellis is using smaller needles. you have an option with cytrellis . let the microcavities open and then the microcavities heals scarlessly without skin tightening or using an elastic membrane for skin tightening. scarring is UNLICKELY to happen with cytrellis .it is your option.... for example women with stretch marks after pregnancy need two things skin tightening and stretch marks removal . so the best option for them is a combination of the first and the second option to remove the stretch marks and tight the skin. even with the second option with skin tightening you can remove completely the scar because the skin itself has plasticity , it can grow and expand ,
I have to mention that cytrellis in their patent says : the microcoring can be combined with scaffolds, and therapeutic agents and drugs......this will be helpful for humans with very poor healing even sunogel's hydrogel can be combined with microcoring but up to normal situations it is not necessary because scarless healing with microcoring will happen of its own
 the image is little small I cant make it big I don't know how, it is from cytrellis patent . microcoring removes full thickness skin (epidermis and the dermis) and you can see how the microcavity heals with new collagen
the image is little small I cant make it big I don't know how, it is from cytrellis patent . microcoring removes full thickness skin (epidermis and the dermis) and you can see how the microcavity heals with new collagen
5 hours ago, damnBOY said:
the image is little small I cant make it big I don't know how, it is from cytrellis patent . microcoring removes full thickness skin (epidermis and the dermis) and you can see how the microcavity heals with new collagen
Do you have a link to the sources your quoting from? Would like to look also.
15 hours ago, damnBOY said:
Please give the link to the source you are linking to. Also, it would be great if you will post all researches you have regarding micro-coring. Thanks.
21 hours ago, damnBOY said:cytrelis patent says:
Technologies, methods, and/or devices described herein can be used for treatment of scars. Scars are characterized by fibroblast proliferation and overexpression of collagen that crosslinks and aligns in one specific direction. Scar tissue is often inferior to healthy tissue, e.g., scars in the skin are less resistant to ultraviolet radiation, lack sweat glands and hair follicles, and are of inferior appearance. Scars can be caused by a variety of conditions, e.g., trauma, abrasion, acne etc., on a variety of tissues. Current treatment of scars include chemical peels, filler injections (e.g. collagen), dermabrasion, laser treatment, radiotherapy, dressing, and steroids, all of which can have significant limitations and/or side effects. Technologies, methods, and/or devices herein can be used to debulk scars by removing microcores from the scar, thus breaking up the hardened tissue and allowing healthy tissue to grow into the microcavities. For example, technologies, methods, and/or devices described herein can be applied to scars on skin or other tissue, such as muscles, e.g., scars on the heart muscle after myocardial infarction.
This sounds very good. What the healthy tissue will look like might not be as it was before the scar though, as it still wont have appendages. However it will be healthy and wont burn as easily or why mention the UV in the patent? I am sure however the healthy tissue will blend into unharmed tissue much better and orientation of collagen improved also. Im going to try it when it comes out as personally I have nothing to loose as I have a surgical scar.
5 hours ago, nikki_gargin said:This sounds very good. What the healthy tissue will look like might not be as it was before the scar though, as it still wont have appendages. However it will be healthy and wont burn as easily or why mention the UV in the patent? I am sure however the healthy tissue will blend into unharmed tissue much better and orientation of collagen improved also. Im going to try it when it comes out as personally I have nothing to loose as I have a surgical scar.
How do you know there will be no appendages?
9 hours ago, nikki_gargin said:This sounds very good. What the healthy tissue will look like might not be as it was before the scar though, as it still wont have appendages. However it will be healthy and wont burn as easily or why mention the UV in the patent? I am sure however the healthy tissue will blend into unharmed tissue much better and orientation of collagen improved also. Im going to try it when it comes out as personally I have nothing to loose as I have a surgical scar.
It will take 100000 + treatments to remove all that scar tissue.If you have the funds and time, you should definitely try it. If you ask me, it's not worth it.
32 minutes ago, MyBeautifulScars said:It will take 100000 + treatments to remove all that scar tissue. If you have the funds and time, you should definitely try it. If you ask me, it's not worth it.
it needs 4-6 treatments, if you do the math , first treatment 25% scar coverage this means you remove 25% of the scar you wait 3 monthes you remove other 25% of the scar you wait 3 monthes another 25% after 4 treatments you will have remove 100% but lets say the last treatments the healthy skin is more than scar and you cant see were is the scar to be sure that you will have remove 100% of the scar you do more two treatments , in total you have removed 150% of the affected area.... the studies says you can remove up to 40% without scarring but lets say you want to 100% safe and you go down to 25% you will need 1-1,5 year to remove every scar from your body. the study says Thirty-two 1 — 1-inch sites per flank received either 20 or 40 percent treatment coverage. Photographs were taken and punch biopsies were performed at days 0, 7, 28, 56, and 84. Biopsy specimens were evaluated for histology and collagen content.
RESULTS:
All treatment sites healed quickly, with no evidence of scarring or infection. Coring sites were easily identified and contained increased fibroblast activity and newly synthesized collagen. At 1 month, the papillary dermis and epidermis of the coring sites were up to 196 percent thicker compared with controls (p < 0.001). The coring sites had enhanced undulating rete ridges-consistent with regeneration. At 3 months, a pronounced increase in collagen fibers and newly organized and augmented elastic fibers was seen. Enzyme-linked immunosorbent assay confirmed an 89 percent increase in collagen content in these coring sites (p < 0.001).
CONCLUSIONS:
This novel approach to skin rejuvenation was found to effectively induce the microscopic and biological endpoints of skin rejuvenation. This may provide a new modality for the safe and cost-effective treatment of age-related rhytides, skin laxity, photodamage,
4 hours ago, PTAA said:How do you know there will be no appendages?
in fact skin appendages can grow in healthy skin does anybody here has so big scars like burn scars with skin grafts and artificial skin I don't know how effective microcoring will be in these situations where affected areas are with artificial skin and skin grafts but for common acne scars , surgical scars , small scars , striae distancae of course it will but in these situation skin appendages are not a big deal you have most of the appendages , sweat glands are going down to hypodermis and the hair root also going down to hypodermis 
1 hour ago, damnBOY said:it needs 4-6 treatments, if you do the math , first treatment 25% scar coverage this means you remove 25% of the scar you wait 3 monthes you remove other 25% of the scar you wait 3 monthes another 25% after 4 treatments you will have remove 100% but lets say the last treatments the healthy skin is more than scar and you cant see were is the scar to be sure that you will have remove 100% of the scar you do more two treatments , in total you have removed 150% of the affected area.... the studies says you can remove up to 40% without scarring but lets say you want to 100% safe and you go down to 25% you will need 1-1,5 year to remove every scar from your body. the study says Thirty-two 1 — 1-inch sites per flank received either 20 or 40 percent treatment coverage. Photographs were taken and punch biopsies were performed at days 0, 7, 28, 56, and 84. Biopsy specimens were evaluated for histology and collagen content.
RESULTS:
All treatment sites healed quickly, with no evidence of scarring or infection. Coring sites were easily identified and contained increased fibroblast activity and newly synthesized collagen. At 1 month, the papillary dermis and epidermis of the coring sites were up to 196 percent thicker compared with controls (p < 0.001). The coring sites had enhanced undulating rete ridges-consistent with regeneration. At 3 months, a pronounced increase in collagen fibers and newly organized and augmented elastic fibers was seen. Enzyme-linked immunosorbent assay confirmed an 89 percent increase in collagen content in these coring sites (p < 0.001).
CONCLUSIONS:
This novel approach to skin rejuvenation was found to effectively induce the microscopic and biological endpoints of skin rejuvenation. This may provide a new modality for the safe and cost-effective treatment of age-related rhytides, skin laxity, photodamage,
in fact skin appendages can grow in healthy skin does anybody here has so big scars like burn scars with skin grafts and artificial skin I don't know how effective microcoring will be in these situations where affected areas are with artificial skin and skin grafts but for common acne scars , surgical scars , small scars , striae distancae of course it will but in these situation skin appendages are not a big deal you have most of the appendages , sweat glands are going down to hypodermis and the hair root also going down to hypodermis
How's SkinTE working out?
16 hours ago, MyBeautifulScars said:It will take 100000 + treatments to remove all that scar tissue.If you have the funds and time, you should definitely try it. If you ask me, it's not worth it.
Nope. It removes0.6mm per microcore. My scar is 1cm wide. You can do the math on that and yes I got the money to pay so why not? I dont expect perfect like some these folk so for me its a no brainier. I want as much fibrotic tissue gone as possible, thats my only goal. I think you will be surprised you know how much it could help if you scars were at least broken down in terms of appearance x
20 hours ago, PTAA said:How do you know there will be no appendages?
Because there is no appendages in fibrotic scar tissue so how micro coringout there scar wil bring back all the glands? Unless the individual still has them within the scar tissue which is possible then how will they magically reappear? Depends on level of damage like dammboy said.
1 hour ago, nikki_gargin said:Nope. It removes0.6mm per microcore. My scar is 1cm wide. You can do the math on that and yes I got the money to pay so why not? I dont expect perfect like some these folk so for me its a no brainier. I want as much fibrotic tissue gone as possible, thats my only goal. I think you will be surprised you know how much it could help if you scars were at least broken down in terms of appearance x
Because there is no appendages in fibrotic scar tissue so how micro coringout there scar wil bring back all the glands? Unless the individual still has them within the scar tissue which is possible then how will they magically reappear? Depends on level of damage like dammboy said.
appendages can grow back in a healthy skin that's why sometimes lasers and microneedling trigger hair follicle formation
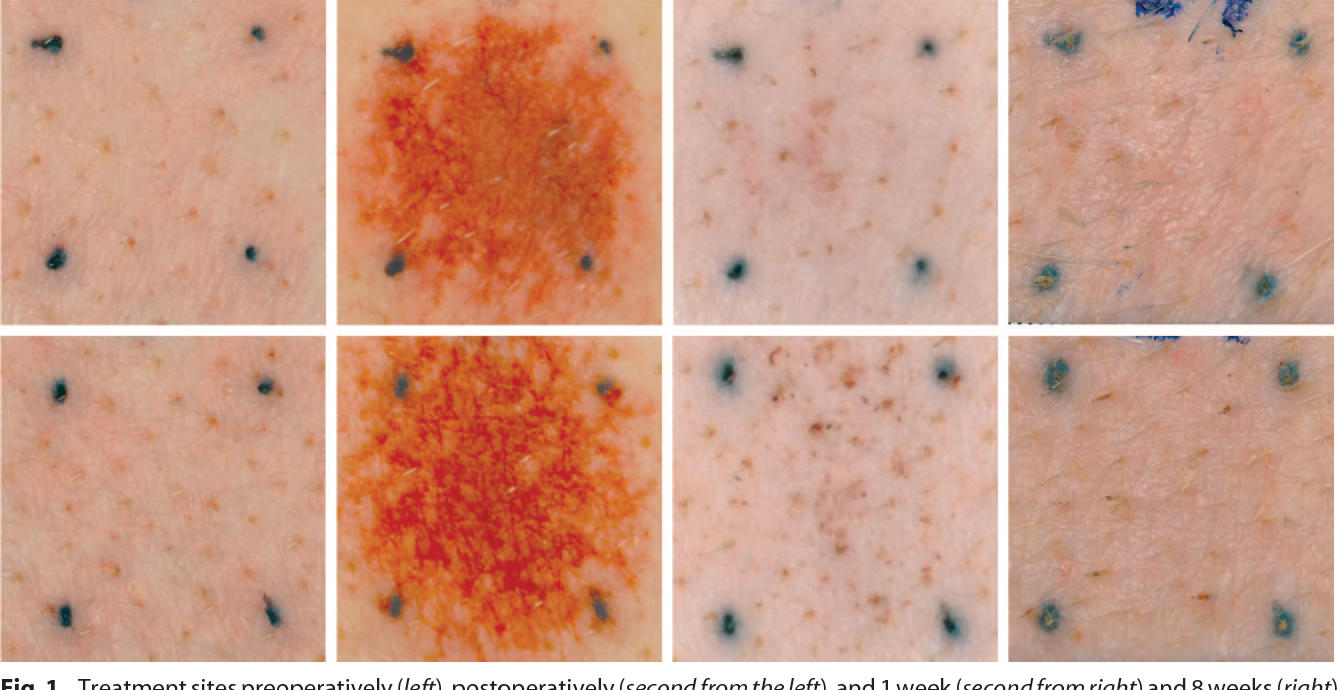
People may find this interesting. Porcine study. If you look closely at the orange freckles you can see they have become wider vs the control. Looking like the skin has stretched also? This looks very good.
Fig. 1. Treatment sites preoperatively (left), postoperatively (second from the left), and 1 week (second from right) and 8 weeks (right) after a 25-gauge needle group at 40 percent coverage: solid needles (above) and coring needles (below).
 Acne.org Products
Acne.org Products