10 hours ago, SunnyX said:Hey,
not all scars are filled by myofibroblast, this is the case with hypertrophic and keloid or contractual scars. Whereas athropic scars are different, they are indented, for example stretch marks, they are not full of fibrotic material which is collagen filled in by myofibroblast. Instead the dermis is lacking the other appendixes and has infact few collagen bundles of hold the skin together. This is also why stretchmarks and athropic are filled in with healthy organized collagen bundle by laser or microneedling. Basically what I wanted to say is that depending on the type of scar a different approach will be required and used. In Stretchmarks and in athropic scar it would be sufficient to inject it or by microneedling it and apply skinte. This approach is also used by Elastagen to apply Tropoelastin. In fact currently also micro fat grafting is used to inject fat within the scar tissue, to break down the collagen bundles and allow the stemcells in fat to sort of regenerate the skin to some extend. This approach will be most probably used to apply it for hair regeneration in bold men and in nerve regenerate which is the next product of polarity. Since they want to apply their approach to multiple tissues for large variety of injuries or cosmetic application, therefore multiple ways of administering will or has to be used... 3rd degree burns all layers have to be removed as done currently as well, in 2degree burns however only the epidermis has to be removed since most of the dermis is intact. So even in burns it won't be applied the same way, so why assume that it can only be applied in a single way. well it won't be done during lunch break since they need a biopsy of the size of a poststamp and that wound has to be treated. Yeah I will let you know as soon I get a feedback.
Yes I do hope you're right with much more simplistic methods, but it would not be a deal breaker if they told me they'd cut my skin to achieve scarless regeneration.
And yes scarring is different. Let me correct my previous statement with this: "The more problematic scars are filled with Myofibroblasts," but that's why I also said they'd probably still have to wound and provide a big enough wound setting to localize the entire area that is to be treated. Micrografting with a punch is exactly what I was referring to in a previous reply to another member and if that is what you were referring to then yes it's exactly what I suggested as well.
No I don't necessarily believe there's only one way the treatment needs to be administered, but if it ends up working perfectly in burns then why not use a similar method for everything else? If they excised the burn wound and applied their product then why not in general excise everything else because it was already proven to work that way? The general idea could be excising, but the method to excise could be different for example: smaller acne or scars regardless of the type could be excised with a punch (micrografting as stated in the last paragraph) of a fitting size where as a nasty long scar could be excised with similar current scar revision methods where it involves making surgical incisions. Just a possibility of course and even if they did start out like that I'm also pretty optimistic they would find less aggressive ways to administer treatment later on although a punch is probably already good enough for many people.
Or this could work as you said and certain conditions can be resolved with a simple prick which would be great and is also a very real possibility. The thing is though, their patent is based on regeneration through wounding. So my only worry would be if the wound setting is big enough or if smaller problems can be addressed as you said and the injection is sufficient enough to create the necessary wound setting for smaller areas. It would make sense that you don't need to wound the skin as much for smaller areas.
Yes some skin conditions do not have any Myofibroblasts, but then wouldn't they have to create them first so that they could then be converted to adipose fat? That's where I would be stumped but then wounding could solve that. Those Myofibroblasts create scar tissue unless instructed to become adipose fat which then creates healthy tissue. That's the reason I assume a big enough wound setting would be necessary where a form of excision can help create and establish that process for conversion and regeneration.
7 hours ago, Frasier said:I agree. Dont overthink too much. If SkinTE can regenerate skin - it can! If it can heal burn wounds scar free how on earth can it be a problem to heal acne scars?? Why shouldnt the doctors want to cut out acne scars if it possible to regenerate the wounds? Too aggressive you say? Well, doctors have been treating acne sufferers with too aggressive lasers for years.
I dont even care about how they will do it. If they need to cut my face, well so be it. I dont even think about going in on a lunch break to regenerate my skin - I could be hospitalized for a week after such treatment. I dont care! I only want SkinTE to work. To me it is kind of "snobby" to be asking for a lunch break treatment to get my skin regenerated.
And your background for saying that is?
I hope you are not under the impression that I demand a lunch break treatment haha. I suggested that since a simple injection may be able to solve some skin conditions and It would be nice if they did achieve that because it would become common everyday practice in today's society, but by all means if they needto slice part of my face for scarless skin regeneration then I'll bring my kitchen knife if I have to lol. Either way those that don't like that possibility will end up coming around eventually anyway.
Eradicate scars safely and make them an insignifcant occurrence is what I'll be satisfied with.
30 minutes ago, 34erer34 said:I am curious as to why SkinTe's projections are so optimistic. idk, hopefully we something soon, by 2020 at least.
You'll know by mid next year. They want to market it in 2018. Thats for burns, but we all know if burns are solved then so will everything else be regarding skin.
cosmetic/scar revisions would follow shortly after if they use the same method by acquiring our own cells which they will because why waste time figuring out an alternative method that would consume time and money when there's no need.
@Tano1Myofibroblast and reprogramming them is the idea into fat cells is the idea of dr Cotsarelis. For that however they need follicle regeneration or bmp. However LGR has been proven to even regrow finger tips, myofibroblast are not necessarily required. The myofibroblast are made of fibroblast, if the wounding is large the myofibroblast will create thick collagen bundles which we call a scar. However, if damage is small then the myofibroblast stays deactivated and eventually cause no scarring. This is the idea behind micro needling and lasers. They cause micro injuries in the skin which the body heals perfectly, this has to do with the communication of cells. If the distance is small they can communicate and migrate and that would cause no scarring. E.g. Small diabetic needles or injection needle punch through the skin but leave no scars behind. Furthermore you have to consider the fact that our skin regenerates itself all the time but the scar tissues remain because the required cells are missing. Now polarity is using this concept it provides all the required cells so that the communication between cells can take place and for the body it seems that it's a small injury so the myofibroblast do not get activated even if by the neogenesis of hairfollicle they will be turned into fat. Well polarity guys have said in an interview that their product will be applicable with current methods and that they are looking at cosmetic application of their product. Furthermore they said that they only released 1% of the data they have gathered and that they want to treat different types of ailments. In one picture in their presentation they also say deployment in burns, wounds and scars. So I guess they mean scars because removal would mean that it's a wound. Also it propagates into old scar tissue without removing it, so microneedling or even multiple injections would be sufficient to treat most scars etc.
5 hours ago, Anish004 said:for my scar anyone suggest a treatment which fill those and match that texture with normal surrounding skin so like nothing was there before..? Skin Graft or needling + stem cell Recell or Acell ??? Anything ?
Skin grafting? What? All you can do is reverse it. Recell now uses needling instead of laser btw. It's around 5k for the kit. Plus accommodations and plane ticket to Perth Australia or Nottingham UK.
From SkinTe's site, not to be a Debbie Downer, but we will be old men by the time any of this hits the market. FDA approval is a long and drawn out process. Recell for example is barely entering the last stage of FDA approval. It can take around 10 years for a product to reach market. Most of you would benefit from just learning to live with yourself.
Certain statements contained in this release are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Forward looking statements contained in this release relate to, among other things, the success of the Company's lead product, SkinTE, the results of future clinical trials and the Company's ability to initiate human clinical trials or the success thereof. They are generally identified by words such as "believes," "may," "expects," "anticipates," "should'" and similar expressions. Readers should not place undue reliance on such forward-looking statements, which are based upon the Company's beliefs and assumptions as of the date of this release. The Company's actual results could differ materially due to risk factors and other items described in more detail in the "Risk Factors" section of the Company's Annual Reports and other filings with the SEC (copies of which may be obtained at www.sec.gov). Subsequent events and developments may cause these forward-looking statements to change. The Company specifically disclaims any obligation or intention to update or revise these forward-looking statements as a result of changed events or circumstances that occur after the date of this release, except as required by applicable law.
8 minutes ago, JohnRottenSkin said:1 hour ago, 34erer34 said:Skin grafting? What? All you can do is reverse it. Recell now uses needling instead of laser btw. It's around 5k for the kit. Plus accommodations and plane ticket to Perth Australia or Nottingham UK.From SkinTe's site, not to be a Debbie Downer, but we will be old men by the time any of this hits the market. FDA approval is a long and drawn out process. Recell for example is barely entering the last stage of FDA approval. It can take around 10 years for a product to reach market. Most of you would benefit from just learning to live with yourself.
Certain statements contained in this release are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Forward looking statements contained in this release relate to, among other things, the success of the Company's lead product, SkinTE, the results of future clinical trials and the Company's ability to initiate human clinical trials or the success thereof. They are generally identified by words such as "believes," "may," "expects," "anticipates," "should'" and similar expressions. Readers should not place undue reliance on such forward-looking statements, which are based upon the Company's beliefs and assumptions as of the date of this release. The Company's actual results could differ materially due to risk factors and other items described in more detail in the "Risk Factors" section of the Company's Annual Reports and other filings with the SEC (copies of which may be obtained at www.sec.gov). Subsequent events and developments may cause these forward-looking statements to change. The Company specifically disclaims any obligation or intention to update or revise these forward-looking statements as a result of changed events or circumstances that occur after the date of this release, except as required by applicable law.
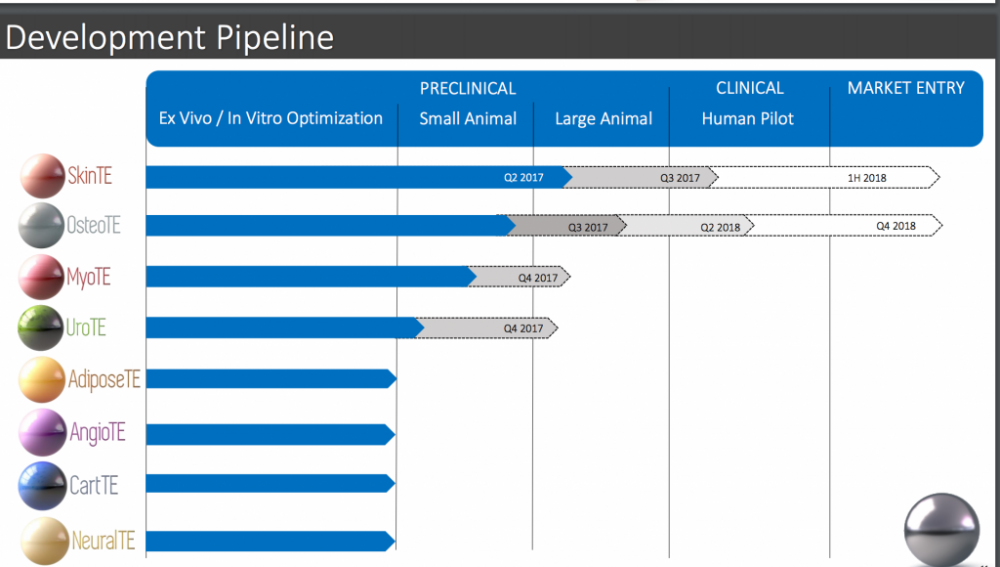
But what means "market entry by 1h 2018"?
First half 2018
8 minutes ago, JohnRottenSkin said:1 hour ago, 34erer34 said:Skin grafting? What? All you can do is reverse it. Recell now uses needling instead of laser btw. It's around 5k for the kit. Plus accommodations and plane ticket to Perth Australia or Nottingham UK.From SkinTe's site, not to be a Debbie Downer, but we will be old men by the time any of this hits the market. FDA approval is a long and drawn out process. Recell for example is barely entering the last stage of FDA approval. It can take around 10 years for a product to reach market. Most of you would benefit from just learning to live with yourself.
Certain statements contained in this release are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Forward looking statements contained in this release relate to, among other things, the success of the Company's lead product, SkinTE, the results of future clinical trials and the Company's ability to initiate human clinical trials or the success thereof. They are generally identified by words such as "believes," "may," "expects," "anticipates," "should'" and similar expressions. Readers should not place undue reliance on such forward-looking statements, which are based upon the Company's beliefs and assumptions as of the date of this release. The Company's actual results could differ materially due to risk factors and other items described in more detail in the "Risk Factors" section of the Company's Annual Reports and other filings with the SEC (copies of which may be obtained at www.sec.gov). Subsequent events and developments may cause these forward-looking statements to change. The Company specifically disclaims any obligation or intention to update or revise these forward-looking statements as a result of changed events or circumstances that occur after the date of this release, except as required by applicable law.
But what means "market entry by 1h 2018"?
First half 2018
it will be on hospitals bro
its autologus and they will need a registration with FDA , no long term clinical trials
if they say that they will bring their product at 2018 and they need long term clinical trials to take approval then polarityTE is a f*cking joke. they dont know what they are doing
1 minute ago, JohnRottenSkin said:yeah. modern fairytales. we need to spam about skinte in any possible medicine site or forum to gather more opinions. Or find polarityte members social accounts, and spam them with questions.
waitng for sunnyx to bring back some positive
hahahahahahah
This link is for anyone who keeps talking about a long FDA process, stop spreading misinformation!
http://medcitynews.com/2017/08/reinventing-tissue-regeneration/
1 hour ago, damnBOY said:it will be on hospitals bro
its autologus and they will need a registration with FDA , no long term clinical trials
if they say that they will bring their product at 2018 and they need long term clinical trials to take approval then polarityTE is a f*cking joke. they dont know what they are doing
Everything has to get FDA approval. Autologous means nothing here. Recell is autologous as well. If they start human trials this year it still has to run a normal clinical trial. That takes the better part of a decade. That is just for burns mind you, fresh burns. Then maybe they move to existing scars, add on another trial for that. Maybe by the time I am 50 0r 60 they'll have something. The unfounded optimism is going to ruin your head dude.
9 hours ago, SunnyX said:@Tano1Myofibroblast and reprogramming them is the idea into fat cells is the idea of dr Cotsarelis. For that however they need follicle regeneration or bmp. However LGR has been proven to even regrow finger tips, myofibroblast are not necessarily required. The myofibroblast are made of fibroblast, if the wounding is large the myofibroblast will create thick collagen bundles which we call a scar. However, if damage is small then the myofibroblast stays deactivated and eventually cause no scarring. This is the idea behind micro needling and lasers. They cause micro injuries in the skin which the body heals perfectly, this has to do with the communication of cells. If the distance is small they can communicate and migrate and that would cause no scarring. E.g. Small diabetic needles or injection needle punch through the skin but leave no scars behind. Furthermore you have to consider the fact that our skin regenerates itself all the time but the scar tissues remain because the required cells are missing. Now polarity is using this concept it provides all the required cells so that the communication between cells can take place and for the body it seems that it's a small injury so the myofibroblast do not get activated even if by the neogenesis of hairfollicle they will be turned into fat. Well polarity guys have said in an interview that their product will be applicable with current methods and that they are looking at cosmetic application of their product. Furthermore they said that they only released 1% of the data they have gathered and that they want to treat different types of ailments. In one picture in their presentation they also say deployment in burns, wounds and scars. So I guess they mean scars because removal would mean that it's a wound. Also it propagates into old scar tissue without removing it, so microneedling or even multiple injections would be sufficient to treat most scars etc.
Ah yes I got off track by mixing Follica's research with PolarityTE. They use the Wnt signaling pathways to aid in regeneration. It does make sense though so hopefully you are right regarding scars. Removal isn't necessary to create wounding. Microneedling is also wounding as well. My issue was I was just not sure if that would create a big enough wound setting or allow it to localize into the entire area for the cells to be administered properly, but if their method can propagate into old scar tissue as well where there is often low blood supply, absence of hair and other tissue, then I can assume something like Microneedling would definitely work.
Scars have the absence of hair follicles, but if they really can achieve follicular regneration inside of present scar tissue and all of it without an excision, then this patentis a lot bigger than everyone thinks. They said they achieved fully functional skin with hair and sweat glands and all that good stuff so I'd assume they would achieve complete regeneration of the skin and its appendages in scar tissue as well.
Perhaps this is why they have already claimed to branch out into scar revisions and hair regeneration. The amount of people to benefit from this will easily reach into the hundreds of millions. Great info!
Get ready guys, I have a feeling this is going to be huge when it drops!
1 hour ago, 34erer34 said:2 hours ago, damnBOY said:it will be on hospitals bro
its autologus and they will need a registration with FDA , no long term clinical trials
if they say that they will bring their product at 2018 and they need long term clinical trials to take approval then polarityTE is a f*cking joke. they dont know what they are doing
Everything has to get FDA approval. Autologous means nothing here. Recell is autologous as well. If they start human trials this year it still has to run a normal clinical trial. That takes the better part of a decade. That is just for burns mind you, fresh burns. Then maybe they move to existing scars, add on another trial for that. Maybe by the time I am 50 0r 60 they'll have something. The unfounded optimism is going to ruin your head dude.
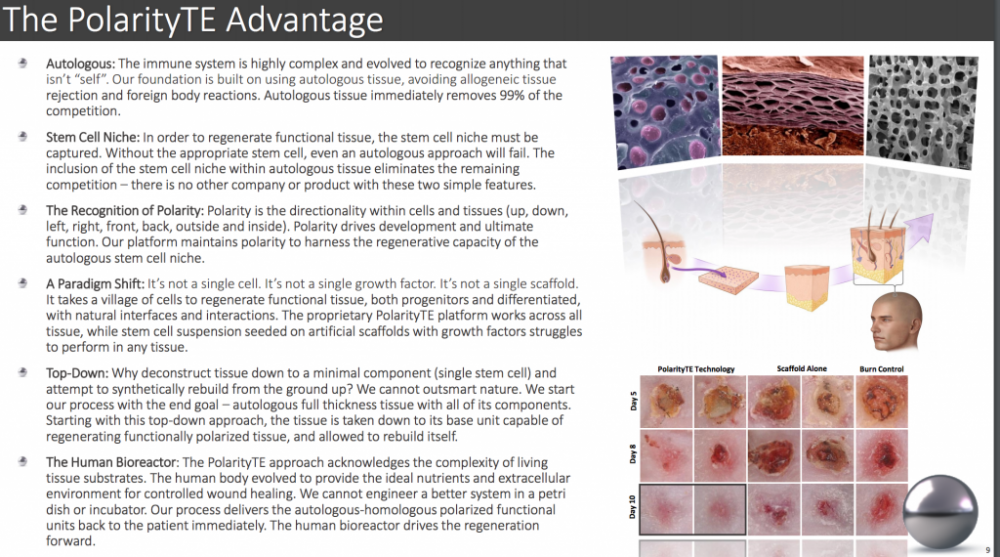
They're not using incubators or scaffolds or external factors. No risk of rejection such as the case in transplanted foreign organs from a different host.
It's all Autologous and Homologous and will also be administered using current already accepted methods of application.
Some phases can be bypassed by FDA depending on the construct you're using. They are indeed doing human trials though, but it is supposed to last a year.
IF successful, this is going to market in 2018, not in decades. It means regular people (not from trials) will have burns treated in 2018 Please see the pictures I've attached below.
It's not a "maybe", they have made it clear that they will go into cosmetic/scar revision. If you understand that they will be using the same method with perhaps a different but also accepted method of application, then you will know they won't go through a long process. In fact, they would probably end up with another year long trial just to make sure it goes as expected and it can arrive to market in 2019 or 2020 at the latest.
3 minutes ago, Tano1 said:They're not using incubators or scaffolds or external factors. No risk of rejection such as the case in transplanted foreign organs from a different host.It's all Autologous and Homologous and will also be administered using current already accepted methods of application.
Some phases can be bypassed by FDA depending on the construct you're using. They are indeed doing human trials though, but it is supposed to last a year.
IF successful, this is going to market in 2018, not in decades. It means regular people (not from trials) will have burns treated in 2018 Please see the pictures I've attached below.
Don't bother trying to explain this to him, he just can't comprehend this...
1 hour ago, mjg713 said:This link is for anyone who keeps talking about a long FDA process, stop spreading misinformation!
http://medcitynews.com/2017/08/reinventing-tissue-regeneration/
I want to believe but will remain skeptical until I hear more.
29 minutes ago, mjg713 said:32 minutes ago, Tano1 said:They're not using incubators or scaffolds or external factors. No risk of rejection such as the case in transplanted foreign organs from a different host.It's all Autologous and Homologous and will also be administered using current already accepted methods of application.
Some phases can be bypassed by FDA depending on the construct you're using. They are indeed doing human trials though, but it is supposed to last a year.
IF successful, this is going to market in 2018, not in decades. It means regular people (not from trials) will have burns treated in 2018 Please see the pictures I've attached below.
Don't bother trying to explain this to him, he just can't comprehend this...
Hey man, I hope it actually happens that fast, it's just not typical is my point. Regardless we will see what happens in time.
1 hour ago, 34erer34 said:3 hours ago, mjg713 said:This link is for anyone who keeps talking about a long FDA process, stop spreading misinformation!
http://medcitynews.com/2017/08/reinventing-tissue-regeneration/I want to believe but will remain skeptical until I hear more.
Then be skeptical, not bombastic.
 Acne.org Products
Acne.org Products