Something nice to read http://www.scientiareview.org/pdfs/240.pdf
Something nice to read http://www.scientiar...rg/pdfs/240.pdf
Nice and interesting! So now there are 4 different technologies that promise a lot in the field of skin regeneration.
And here is something about technological progress in general - price of the genome sequencing - it was $3 billion in 1990, $100 million in 2001, $10K in 2012. and it will be only $1K in 2014:

All scarring is, is an over growth of collagen that blocks off regeneration thats it.
hey seabs
in your opinion...
do you think that hydrogel works on keloids?
You must investigate more bout keloids...many people have that cain of scar (keloid like all scar is an over growth of collagen path in keloids are more problems implicated like an "non stop" signal (non stop make collagen), a decrease of tgf b1,and b2 (perhaps there are the non stop signals).
please investigate that cain of scar.
All scarring is, is an over growth of collagen that blocks off regeneration thats it.
hey seabs
in your opinion...
do you think that hydrogel works on keloids?
You must investigate more bout keloids...many people have that cain of scar (keloid like all scar is an over growth of collagen path in keloids are more problems implicated like an "non stop" signal (non stop make collagen), a decrease of tgf b1,and b2 (perhaps there are the non stop signals).
please investigate that cain of scar.
A keloid is a collagen overgrowth like anyother scar. From the facts I've seen, all a scar is, is a collagen overgrowth in a constant feed back loop, were the fibroblasts are continuing to lay down the bundles of collagen to and beyond 30 days. Though keloids are way more extensive. Logic says, if the body reepithilizes normal tissue faster (in under 21 days) than the feed back loop then there will be no scar.
New article found on pubmed
http://www.ncbi.nlm....pubmed/22687479
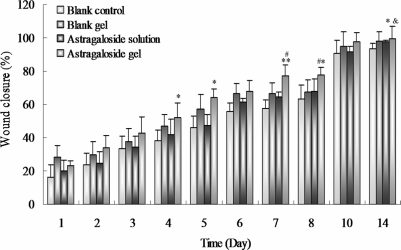
Results of this study provided evidence for the alginate-gelatin hydrogel as efficient carrier for the topical delivery of bioactive molecules to the injured site. The astragaloside IV releasing hydrogel was shown a promising therapeutic formulation for wound healing, as well as its regenerative feature and underlying mechanism contribute to the skin regeneration were disclaimed.
(the full article is still in process)
New article found on pubmed
http://www.ncbi.nlm....pubmed/22687479
Results of this study provided evidence for the alginate-gelatin hydrogel as efficient carrier for the topical delivery of bioactive molecules to the injured site. The astragaloside IV releasing hydrogel was shown a promising therapeutic formulation for wound healing, as well as its regenerative feature and underlying mechanism contribute to the skin regeneration were disclaimed.
(the full article is still in process)
You can already get the full article if you navigate to the first link under the "link to - more resources" link. By the way, correct me if I'm wrong but, this hydrogel seems to be a different hydrogel than the one mentioned in the previous articles. This quote is taken from the full article:
"In this study, the hydrogel made from the blended materials
of sodium alginate and gelatin was prepared and characterized"
New article found on pubmed
http://www.ncbi.nlm....pubmed/22687479
Results of this study provided evidence for the alginate-gelatin hydrogel as efficient carrier for the topical delivery of bioactive molecules to the injured site. The astragaloside IV releasing hydrogel was shown a promising therapeutic formulation for wound healing, as well as its regenerative feature and underlying mechanism contribute to the skin regeneration were disclaimed.
(the full article is still in process)
You can already get the full article if you navigate to the first link under the "link to - more resources" link. By the way, correct me if I'm wrong but, this hydrogel seems to be a different hydrogel than the one mentioned in the previous articles. This quote is taken from the full article:
"In this study, the hydrogel made from the blended materials
of sodium alginate and gelatin was prepared and characterized"
Thanks
I know it is a different hydrogel. At the moment I have read of 4 different kinds of hydrogel for scarring alone. There is a lot of competition for hydrogel scaffolds on the future market.
The results show that a hydrogel scaffold has two features.
1. It is a great carrier
for topical delivery of bioactive molecules.
2. It increases skin regeneration.
This isn't scarefree healing yet, with al the topicals it can deliver. (inculding stem cells) And the perfection of the scaffold I wonder if something very close to scar free healing can be achieved.
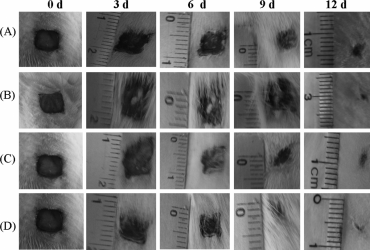
The wound at day 12 is almost completly healed. D=hydrogel.
http://www.scielo.br...ipt=sci_arttext
Impressive results in scar healing with stem cells. (not scar free)
I hadn't seen these yet.
Nice results! But with those exclusion criteria they admit they cannot improve neither hypetrophic scars nor keloids:
The exclusion criteria were smoking, history of keloids or hypertrophic scarring, diabetes mellitus, any skin or connective tissue disease, previous supraumbilical scar, prolonged use of corticosteroids, previous chemotherapy or radiotherapy, weight loss post-obesity, infection, hematoma, seroma or dehiscence during the abdominoplasty postoperative period and patient withdrawal during the course of the study.
Any update on Gerecht hydrogel?
Last time I remember they had problems with funding, seems stange after the whole hype all over the news.
(did the mouse die or turn into a vampire)
Last I heard it was waiting for funding, So since december, that is six and a half month waiting for funding added ontop of the 2.5 year+ it takes for a device to go through the hoops.
One thing bothers me about Sharon Gerecht - is she planning to set up her own company? I'm asking it because I've just read that she has patented her invention
http://releases.jhu....scar-free-skin/
The Johns Hopkins Technology Transfer staff has filed a provisional patent application to protect the intellectual property involved in this project.
so no one else can't use it now and sell it as a scar free treatment in the future, and if that dextran hydrogel is really able to cope with 3rd degree burn wounds, full-thickness skin excisions, diabetic foot ulcers, hypertrophic scars and keloids then she could easily get funding for her company from the venture capital firms and she could make a big fortune (you should remember that Renovo's market value was $500 million in 2006 and 2007 after IPO on the London Stock Exchange and before Juvista's clinical trial failures and the US market size alone for scar improvements was estimated at $4 billion a year)
Here you can see that venture capital funds in the US invest $5 to $7 billion in each quarter in entrepreneurial innovations and scientific inventions, in good business ideas, in anything that is promising and has a market potential
https://www.pwcmoney...page=historical
and most of that money is invested in life sciences and IT industry - and her invention belongs to life sciences sector and appears to be promising and has the huge market potential

I would like to see her as a millionaire but not like this:
http://educationalst...acle/#more-1224
Renovo attempted to commercialise the scientific research that Ferguson had carried out at the University of Manchester. It grew rapidly from a small private company in 2000 to a publicly traded company with over 200 staff, claiming possession of the most advanced regenerative medicine in the world. It received large sums of money, including 63 million of investors money, 58 million of investment from the pharmaceutical company Shire,along with 16.5 million of British tax-payers money in the form of grants and research tax credits.
The Clinical TrialsJuvista succeeded in early phase one and two clinical trials for efficacy but in 2007 a phase two trial testing it to heal scars after mole removal was a failure (Renovo 2007, p10). A 2008 phase two clinical trial using Juvista to heal scars after breast augmentation surgery also failed as differences between the treated scars and the placebo were not significant (Renovo 2008, p7). Juvista did succeed in trials concerning patient safety and Ferguson assured investors that the failed trials were down to the use of sub-optimal doses and a sub-optimal trial design involving scars of different lengths and anatomical locations (Renovo 2009, p7).
Zesteem was another product designed to accelerate wound healing. It made some progress in early trials, but in 2008 Renovos phase three clinical trial for Zesteem failed to meet its primary endpoint for efficacy and all development on it was terminated (Renovo 2008, p8).
Judivex was a treatment that hoped to improve scarring and accelerate re-epithelialisation. However, in 2009, the phase two clinical trial for Judivex failed as the treatment showed no demonstration of a statistically significant difference in the time to complete wound closure (Renovo 2009, p8).
Another drug, Adaprev was also developed. Renovo hypothesised that this injectable formulation would prevent scarring and improve function following surgical repair of lacerated tendons. The trial results were announced in November 2011 and showed that patients treated with Adaprev had less range of motion than those in the standard care group (Renovo 2011, p2). The development of the drug was cancelled.
Prevascar aimed to prevent scarring and restore function after peripheral nerve injury and was in the early phase of trials (Renovo 2006, p9). The trial results were announced on 16 April 2012: the drug failed badly as by month thirteen there was a small but significant improvement in the placebo scar width when compared to the treatment scar.
The Sales Pitch
Renovo never produced a single marketable product, never generated any product revenue and failed in its commercialisation of Fergusons alligator research. Indeed, it burned over 100 million of private and state investment. Driving this process was a constant sales pitch about the potential money to be made.
The WinnersBetween 2006 and 2011 the total paid to Renovos board of directors (including termination payments) was over 11 million (Renovo 2006 to 2011). Mark Ferguson was the highest earner with a total of 3.6 million over the five years. This included bonuses of 971,000 and a payment of 700,000 that was made in June 2011 when his contract as chief executive officer was terminated (Renovo 2011, p17). Renovo also paid an additional total amount of 451,118 into his pension fund over the five years (Renovo 2006 to 2011).
Sharon OKane earned a total of 1.6 million over her four years at the company (Renovo 2006 to 2011). This included a post-cessation payment of 200,488 on her resignation as chief scientific officer in February 2010 (Renovo 2010, p36).
In 2007, Ferguson and OKane also made huge gains by exercising Renovo share options. On 26 June, under the directors share option scheme, Ferguson and OKane acquired over 4.7 million Renovo shares for .004 pence per share (Renovo 2007, p35). They then sold them on to investors at the market price of 2 per share on 2 July (Renovo, 2007). The company had announced the Shire licensing deal on 20 June 2007 and the shares were at their zenith. In a single trade, Ferguson was enriched by 5.9 million and OKane by 3.5 million.
The aggregate scale of the directors pay and the share rewards is remarkable in light of the fact that Renovo never successfully commercialised a product from any of the drug developments.
So all in all Mark Ferguson and his wife have earned about 16 million in five years.
I don't understand why all the BS.
If someone could contact Gerecht in a professional manner we would know more and maybe get more involved.
I would be great to have a genuine scientist on this topic.
Anyway this forum is so large it could use a psychologist, a dermatologist, a skin surgeon etc.
If I where a student in one of these, i would use this topix just to help some folk, inform even as a training.
Kind of sick of all this waiting. I hope Recell brings some great results or else the coming years is going to be hel.
I would be happy to wait another 3 years if I knew for certain it would work. The results seem to be al put on a single mouse.
I hope the mouse is happy with his results and feels confident now. ![]()
Maybe the mouse suddenly exploded after a month.
Using the cited info in that blog I think its reasonable for the blogger to state Renovo looks suspiciously like a scam.
I noted something wasn't right three years ago, when I noticed the result of the phase two trial was seriously poor and there were treatments already out that were better. But because people were mesmerised by the authority and blindly appealed to the authority in a cult like hypnotic state there was no point in debating with a cult.
Juvista was an injection (an injection of anything will never bring scar free healing, your body can only bring scar free healing) that imo was pushed by someone in a white coat, explaining "detailed theory over results" when it should be results over detailed theory, and claiming authority that people blindly followed even after the phase two trials fell flat on their ass.
Scaffolds are not injections. All they do is get digested by the whiteblood cells near the tissue, after they are digested the body does the healing automatically, based on the speed of of its digestion. eg. slow digestion, or rejection of the scaffold brings scar. Fast digestion brings reepithilisation and regeneration.
Well now I know what do you people mean when you say 'scam', I know it because I see that Ferguson and his wife exercised their stock options on 2 July 2007 - just a few days after they have bought it, they didn't want to wait - if the phase II and III trials were sucessfull then their stocks would be worth even more, then they could earn 15 or 20 million or something like that - but they didn't want to hold it until phase II i III clinical trial results were announced, they knew that stock price was at its peak at that moment right after the deal with Shire.
Avita Recell is doing a huge research on burn / hypertrophic / dyspigmented scars.
I have no idea when the results will be published, it could take up to 6 months.
If it is proven effective, this could be the best treatment available today.
Interesting news from RXi Pharmaceuticals, a company founded by Craig Mello - Nobel Prize winner for medicine in 2006 for his discovery of RNAi gene-silencing technology, their drug that is called RXi-109 is an inhibitor of CTGF protein (I guess it is similar to EXC-001 and Excaliard Pharmaceuticals), I hope it is not a scam like Renovo and Juvista
May 31, 2012
RXi Pharmaceuticals Receives FDA Clearance to Begin Clinical Trial with RXI-109
http://www.rxipharma.com/2012/05/3931/
June 26, 2012
RXi Pharmaceuticals Announces Initiation of First Clinical Trial
http://www.rxipharma...clinical-trial/
About RXi Pharmaceuticals
RXi Pharmaceuticals Corporation (OTCBB:RXII) is a biotechnology company focused on discovering, developing and commercializing innovative therapies based on its proprietary, next-generation RNAi platform. Therapeutics that use RNA interference, or RNAi, have great promise because of their ability to silence, or down-regulate, the expression of a specific gene that may be overexpressed in a disease condition. Building on the pioneering work of scientific founder and Nobel Laureate Dr. Craig Mello, RXis first RNAi product candidate, RXI-109, which targets CTGF (connective tissue growth factor), will commence human clinical trials in anti-scarring in 2012.
Craig C. Mello, Ph.D., Founder and Scientific Advisory Board Chairman
Dr. Mello has served as the Chairman of our Scientific Advisory Board since February 2007. Dr. Mello, co-recipient of the 2006 Nobel Prize in Medicine for RNAi, co-discovered RNAi and co-invented RNAi therapeutics. Dr. Mello is the Blais University Chair in Molecular Medicine at the University of Massachusetts Medical School, a Howard Hughes Investigator and a member of the National Academy of Sciences. In 2006, he was named the inaugural recipient of The Dr. Paul Janssen Award for Biomedical Research by Johnson & Johnson and was the co-recipient of the Paul Ehrlich and Ludwig Darmstaedter Prize. Dr. Mello was also the co-recipient of the National Academy of Sciences Award in Molecular Biology and the Wiley Prize in the Biomedical Sciences from Rockefeller University in 2003. He was a postdoctoral fellow at the Fred Hutchinson Cancer Research Center and in 1995, was named a Pew Scholar in the Biomedical Sciences. Dr. Mello received his B.S. in Biochemistry from Brown University in 1982 and his Ph.D. in Cellular and Developmental Biology from Harvard University in 1990.
Interesting news from RXi Pharmaceuticals, a company founded by Craig Mello - Nobel Prize winner for medicine in 2006 for his discovery of RNAi gene-silencing technology, their drug that is called RXi-109 is an inhibitor of CTGF protein (I guess it is similar to EXC-001 and Excaliard Pharmaceuticals), I hope it is not a scam like Renovo and Juvista
May 31, 2012
RXi Pharmaceuticals Receives FDA Clearance to Begin Clinical Trial with RXI-109
http://www.rxipharma.com/2012/05/3931/
June 26, 2012
RXi Pharmaceuticals Announces Initiation of First Clinical Trial
http://www.rxipharma...clinical-trial/
About RXi Pharmaceuticals
RXi Pharmaceuticals Corporation (OTCBB:RXII) is a biotechnology company focused on discovering, developing and commercializing innovative therapies based on its proprietary, next-generation RNAi platform. Therapeutics that use RNA interference, or RNAi, have great promise because of their ability to silence, or down-regulate, the expression of a specific gene that may be overexpressed in a disease condition. Building on the pioneering work of scientific founder and Nobel Laureate Dr. Craig Mello, RXis first RNAi product candidate, RXI-109, which targets CTGF (connective tissue growth factor), will commence human clinical trials in anti-scarring in 2012.
Craig C. Mello, Ph.D., Founder and Scientific Advisory Board Chairman
Dr. Mello has served as the Chairman of our Scientific Advisory Board since February 2007. Dr. Mello, co-recipient of the 2006 Nobel Prize in Medicine for RNAi, co-discovered RNAi and co-invented RNAi therapeutics. Dr. Mello is the Blais University Chair in Molecular Medicine at the University of Massachusetts Medical School, a Howard Hughes Investigator and a member of the National Academy of Sciences. In 2006, he was named the inaugural recipient of The Dr. Paul Janssen Award for Biomedical Research by Johnson & Johnson and was the co-recipient of the Paul Ehrlich and Ludwig Darmstaedter Prize. Dr. Mello was also the co-recipient of the National Academy of Sciences Award in Molecular Biology and the Wiley Prize in the Biomedical Sciences from Rockefeller University in 2003. He was a postdoctoral fellow at the Fred Hutchinson Cancer Research Center and in 1995, was named a Pew Scholar in the Biomedical Sciences. Dr. Mello received his B.S. in Biochemistry from Brown University in 1982 and his Ph.D. in Cellular and Developmental Biology from Harvard University in 1990.
Interesting news from RXi Pharmaceuticals, a company founded by Craig Mello - Nobel Prize winner for medicine in 2006 for his discovery of RNAi gene-silencing technology, their drug that is called RXi-109 is an inhibitor of CTGF protein (I guess it is similar to EXC-001 and Excaliard Pharmaceuticals), I hope it is not a scam like Renovo and Juvista
May 31, 2012
RXi Pharmaceuticals Receives FDA Clearance to Begin Clinical Trial with RXI-109
http://www.rxipharma.com/2012/05/3931/
June 26, 2012
RXi Pharmaceuticals Announces Initiation of First Clinical Trial
http://www.rxipharma...clinical-trial/
About RXi Pharmaceuticals
RXi Pharmaceuticals Corporation (OTCBB:RXII) is a biotechnology company focused on discovering, developing and commercializing innovative therapies based on its proprietary, next-generation RNAi platform. Therapeutics that use RNA interference, or RNAi, have great promise because of their ability to silence, or down-regulate, the expression of a specific gene that may be overexpressed in a disease condition. Building on the pioneering work of scientific founder and Nobel Laureate Dr. Craig Mello, RXis first RNAi product candidate, RXI-109, which targets CTGF (connective tissue growth factor), will commence human clinical trials in anti-scarring in 2012.
Craig C. Mello, Ph.D., Founder and Scientific Advisory Board Chairman
Dr. Mello has served as the Chairman of our Scientific Advisory Board since February 2007. Dr. Mello, co-recipient of the 2006 Nobel Prize in Medicine for RNAi, co-discovered RNAi and co-invented RNAi therapeutics. Dr. Mello is the Blais University Chair in Molecular Medicine at the University of Massachusetts Medical School, a Howard Hughes Investigator and a member of the National Academy of Sciences. In 2006, he was named the inaugural recipient of The Dr. Paul Janssen Award for Biomedical Research by Johnson & Johnson and was the co-recipient of the Paul Ehrlich and Ludwig Darmstaedter Prize. Dr. Mello was also the co-recipient of the National Academy of Sciences Award in Molecular Biology and the Wiley Prize in the Biomedical Sciences from Rockefeller University in 2003. He was a postdoctoral fellow at the Fred Hutchinson Cancer Research Center and in 1995, was named a Pew Scholar in the Biomedical Sciences. Dr. Mello received his B.S. in Biochemistry from Brown University in 1982 and his Ph.D. in Cellular and Developmental Biology from Harvard University in 1990.
That is great news!! If it is not a scam.
A drug that works internally, hydrogel, recell, stem cell, acell, etc. Scareless healing is a matter of time, not possibility!!!!
That is great news!! If it is not a scam.
A drug that works internally, hydrogel, recell, stem cell, acell, etc. Scareless healing is a matter of time, not possibility!!!!
Well I don't know WinnieTheBlue, we should ask Seabs what does he think about that?
I'm not sure that they will achieve some extraordinary good results that are close to scar free healing by targeting only a single gene... I believe they will need a whole pipeline of anti-scarring drugs for the good results... and I don't like to hear 'scarless healing' because if someone say so most probably it's a scam or it means poor results, I prefer to hear 'scar-free healing'... and because of that I have more faith in that hydrogel stuff from John Hopkins University and in that salamander stuff from University of Kentucky and University of Florida - they both say 'scar-free healing' and not 'scarless healing'... but unfortunately both are only research projects at universities... at least for now... and still there are no established companies trying to commercialize scar-free healing therapies that are based on their researches, there are only research papers for now and nothing more...
actually the most interesting thing for me in the whole story from their website is RNAi technology - I think it is one of the most important and most promising discovery in genetic engineering so far - downregulation and upregulation of genes and their expressions is possible without affecting DNA - Craig Mello got Nobel Prize for that discovery in 2006:
http://en.wikipedia.org/wiki/RNAi
http://en.wikipedia....ogy_or_Medicine
that technology could be used not only for scars but for many other diseases.
What I say is always stick to fact based logic, always stick to known logical frameworks, always look for results before anything, like over detailed theory and stick to the results. Do not promote an authority figure, its ok to reference though. Learn from the history of the subject.
 Acne.org Products
Acne.org Products