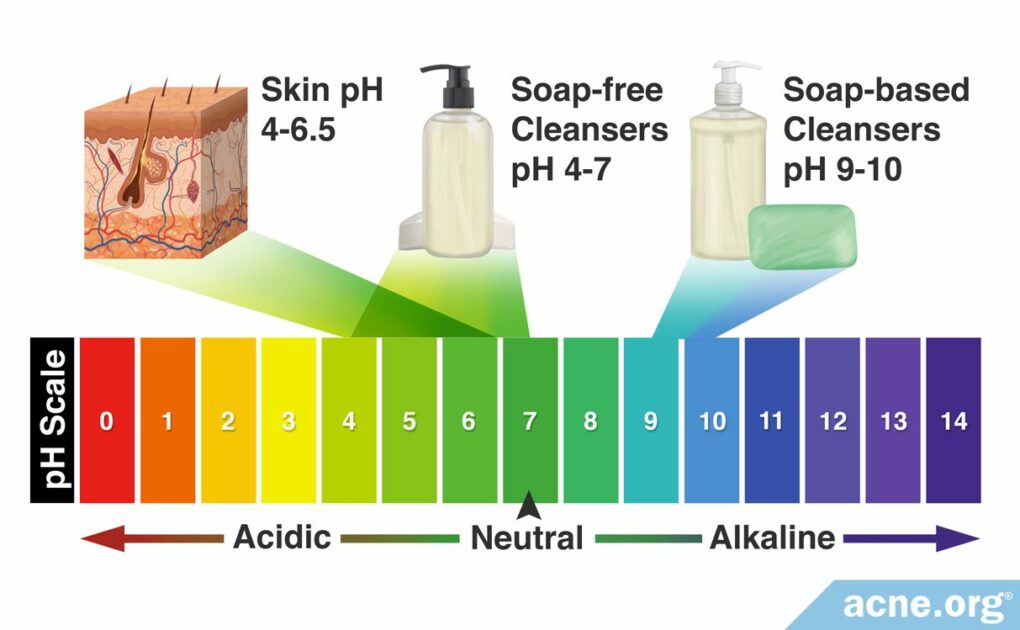
Yes. Choose a Slightly-acidic Cleanser to Avoid Worsening of Acne.

The Essential Info
What Is pH? pH is the measure of how acidic or alkaline (basic) something is.
The pH Scale: On the pH scale that runs from 0-14, a value of 7 is considered neutral. The lower the number the more acidic something is, and the higher the number the more alkaline something is.
Human Skin pH: Human skin tends to have a pH between 4 – 6.5, which means it is slightly acidic.
To Keep Acne at Bay, Keep the Skin Acidic: Sustaining the skin’s slightly acidic pH is necessary for proper skin function and can help keep acne under control. Certain cleansers, and soap in particular, tend to be overly alkaline, and force the skin outside of its natural pH range, which may promote the growth of bacteria and might encourage more acne.
How to Choose a Slightly-acidic Cleanser: Luckily, this isn’t difficult. To ensure that you are using a slightly acidic cleanser, be sure to use only cleansers made specifically for the face and that claim on the label that they are “ultra-gentle,” “made for sensitive skin,” or similar claims. This usually means they are slightly acidic. Always avoid soap.

The Science
- The pH Scale
- Importance of Maintaining a Normal Skin pH Level
- Effect of Cleansers’ pH Value
- Effect of Cleansers’ pH Value on Skin pH Level
- The Bottom Line
Every cleanser possesses a certain pH, which is the measure of its acidity or alkalinity. The pH of a cleanser may be an important quality that controls how it may affect acne.
The pH Scale
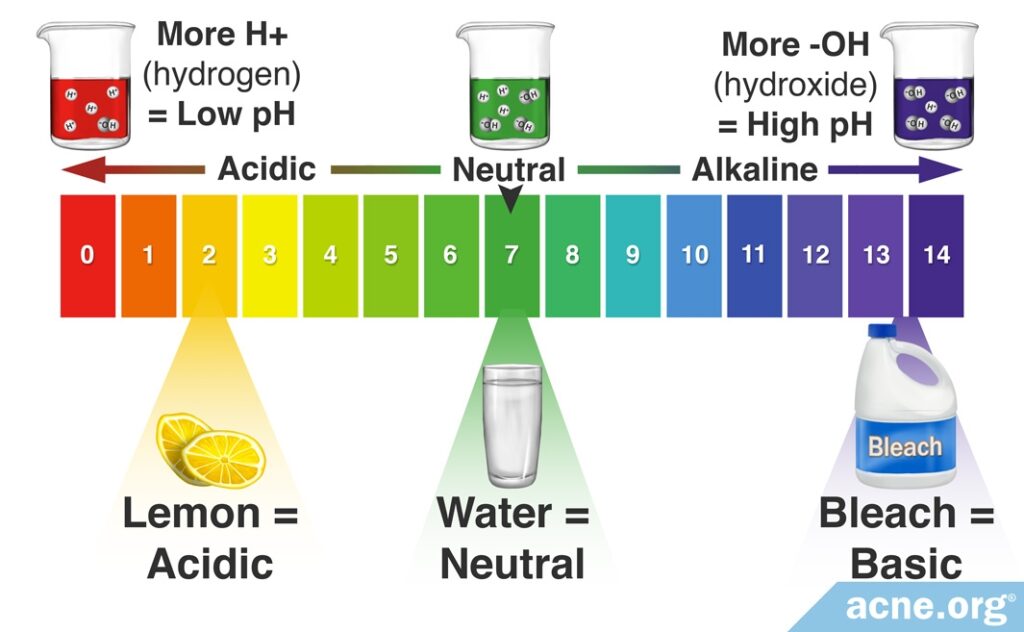
Scientists use the pH scale, which ranges from 0-14 to measure acidity or alkalinity. Water has a pH of 7, which is considered neutral. The more acidic a solution is, the lower is its pH level. Conversely, the more basic or alkaline the solution is, the higher is its pH level. This scale is a logarithmic scale, meaning that there is a 10-fold difference in acidity between each number in the scale. For example, lemon juice – which possesses a pH of 2 – is 10 times more acidic than a solution with a pH of 3 and 10 billion times more acidic than a bleach solution with a pH of 12.
Scientifically speaking, the pH scale represents the number of hydrogen ions in a solution. An acidic solution is one that contains many hydrogen ions (H+), whereas an alkaline solution contains few hydrogen ions.1
Importance of Maintaining a Normal Skin pH Level
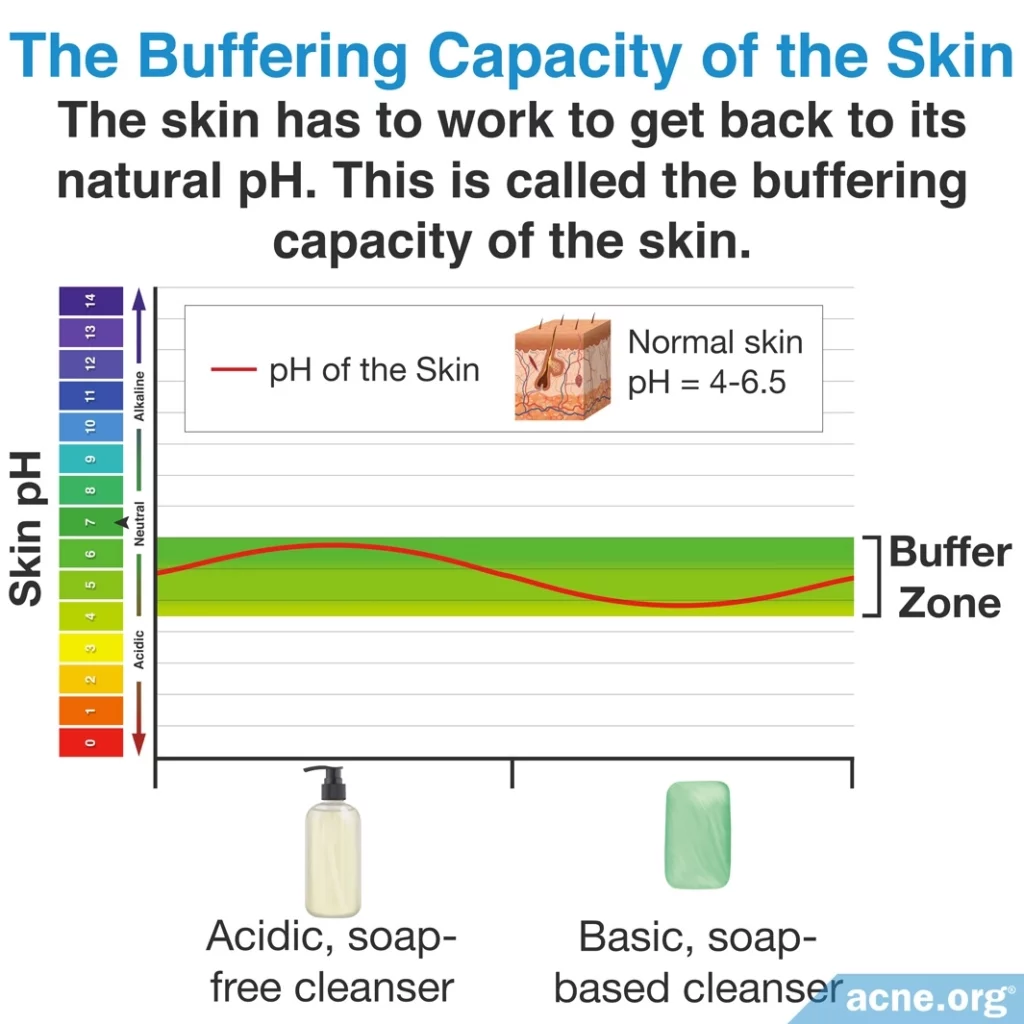
The skin also has its own unique pH, which in humans ranges from 4 – 6.5. Research has shown that maintaining the skin pH “in an acid range of 4 – 6.5 is important for the overall health, integrity, and function” of the skin.2 Disruption of the skin’s pH, through things like using a cleanser that does not have a similar pH to the skin, can lead to dryness and irritation3-5, which may aggravate acne.6
The skin is not completely at the mercy of cleansers, however. When something is put on the skin with a pH that does not match the slightly acidic pH of the skin, the skin uses its powerful buffering capacity, which allows it to resist changes in pH. However, this buffering capacity is only so strong. Prolonged use of very acidic or very alkaline cleansers can overwhelm the skin’s buffering capacity, causing the skin to become overly irritated, and forcing the body to rely on its wound-healing processes to restore the skin’s pH.5 Since we know that anything that irritates the skin can lead to more acne, it is best to use cleansers that mimic the slight acidity of the skin.
Effect of Cleansers’ pH Value
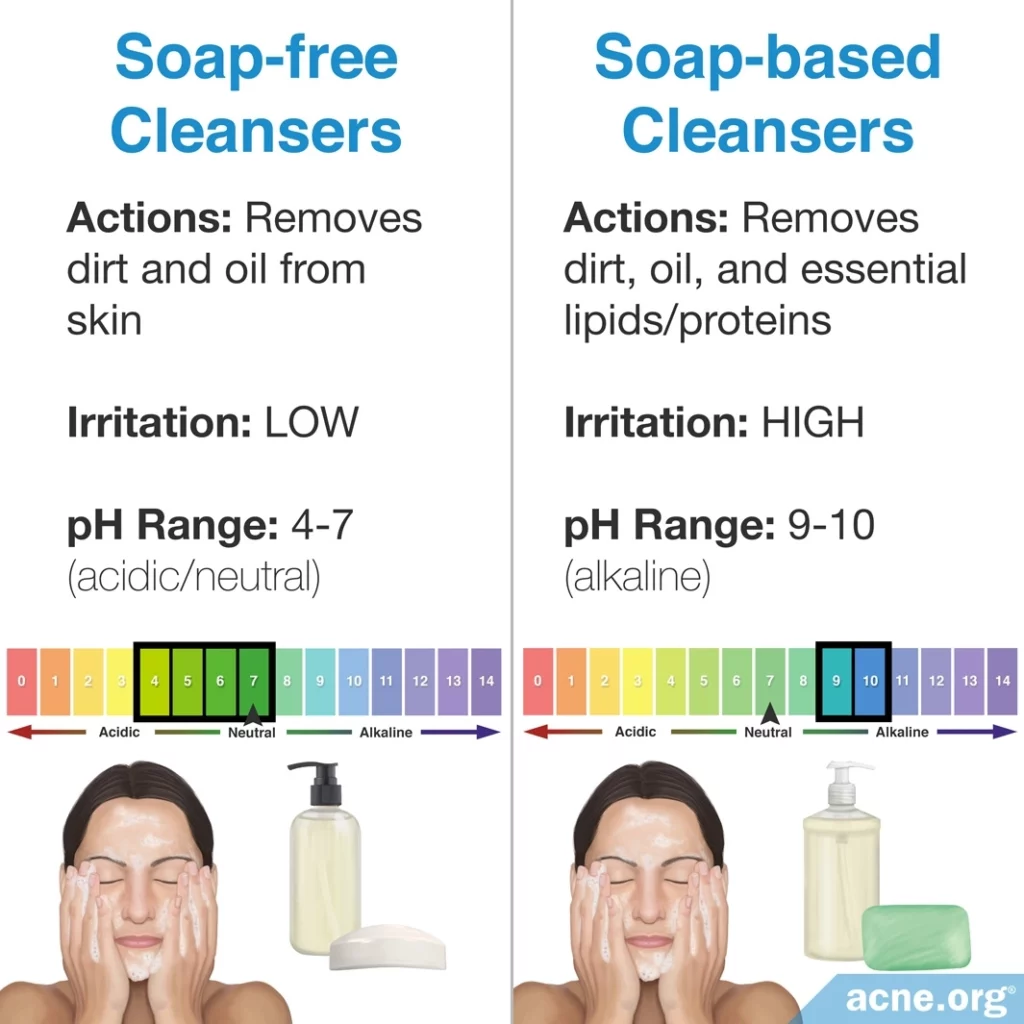
All cleansers have a pH. Normally, most cleansers designed specifically for the face are neutral or slightly acidic and are not of concern. However, soap-based cleansers are basic and can cause problems. When it comes to cleansing products, there are two categories: soap-based cleansers and soap-free cleansers.
Soap-based cleansers
Soap is a basic substance with a pH of 9 – 10. With such a basic pH, soap is harsh because it removes not only dirt and oil from the skin but also removes essential skin fats and proteins, disrupting the skin’s barrier, and resulting in irritation.
In addition, pushing the skin to a higher pH, particularly when this is done repeatedly, allows bacteria to multiply. Since acne is in part a bacterial disease, this is another reason not to use soap-based cleansers.
In line with the idea that a higher pH favors bacteria, one study found that C. acnes, the type of bacteria that plays a role in acne, grows well at a pH of 6 – 6.5 but grows poorly at a more acidic pH.7
Soap-based cleansers either come in bar form (such as Zest, Irish Spring, or Lever 2000) and are normally for washing the body, or come in liquid form (such as Dial) and are made for washing the hands. Another tell-tale sign that you are using a soap-based cleanser is the simple mention of “soap” on the label. Avoid all of these products on acne-prone areas of the body.
Soap-free cleansers
Generally, the pH of soap-free cleansers is neutral (7) or slightly acidic (4 – 6), so soap-free cleansers are less irritating than soap-based cleansers. Dermatologists believe that “maintaining the skin surface at its physiological pH (4 – 6.5) during cleansing prevents overgrowth of certain microorganisms” and that cleansers with a neutral or acidic pH “may be potentially less damaging to the skin.”6,8 Therefore, soap-free cleansers are preferred.
The safest way to find a soap-free cleanser is to look for a liquid cleanser specifically made for the face (such as Cetaphil Gentle Skin Cleanser or Acne.org Cleanser).
Effect of Cleansers’ pH Value on Skin pH Level
Although dermatologists believe that patients should use slightly acidic cleansers to maintain the skin pH and therefore skin health, the research supporting this idea still remains somewhat contradictory. However, some studies point toward the possibility that an increased skin pH might encourage the growth of bacteria. And remember, since acne is partly a bacterial disease, this is something to be avoided.
Expand to read details of studies

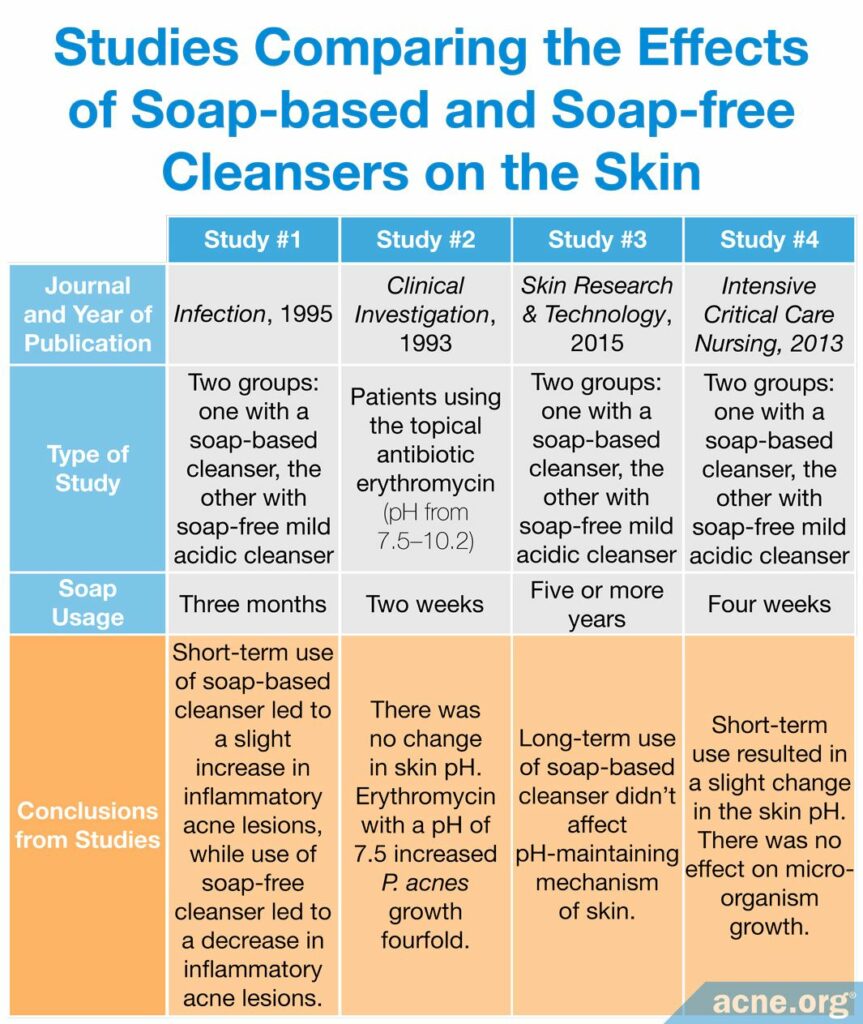
A study published in 1995 in the journal Infection tested the effects of alkaline soap compared to acidic syndet bar in 120 adolescents with acne. The authors found that, from 4 weeks of use onward, the number of inflammatory acne lesions increased somewhat in the teens using the alkaline soap, while it decreased in the teens using the acidic syndet bar. Moreover, the teens using the alkaline soap experienced much more skin irritation compared to the teens who used the acidic syndet bar.9

Another study, published in Clinical Investigation in 1993, examined patients using the topical antibiotic erythromycin at pHs ranging from 7.5 – 10.2 for two weeks. This study found that although there was no change in the skin pH, patients using the erythromycin product at a pH of 7.5 experienced a fourfold growth of C. acnes compared to those without treatment, suggesting that this pH counteracted the antibacterial effect of the product.10

One study, published in the journal Skin Research and Technology in 2015, compared two groups of people who had been using either a soap-based cleanser or soap-free mild acidic cleanser for more than five years. The researchers found that “long-term continuous use of a soap-based cleanser does not affect the pH-maintaining mechanism of human skin.”11

Another study, published in Intensive and Critical Care Nursing in 2013, compared two groups of people who had been using either a soap-based cleanser or soap-free mild acidic cleanser for only four weeks and found that short-term use did result in a slight change in the skin pH. This study also looked at bacteria growth and found that there was no effect on micro-organism growth. This shows that products do not need to be high in pH to hinder bacterial growth, including colonization of the acne-associated bacteria C. acnes. Consequently, washing with harsh soap should be avoided since it is not better for removing bacteria in the skin.12

A review article published in 2013 in the journal Acta Dermato-Venereologica reviewed these studies and concluded: “Use of proper soaps and over-the counter creams that do not compromise the acidic pH of skin should become part of the treatment regimen of [acne] patients. In recommending the ideal body wash, soap or cleanser, one that has a pH between 4.5 – 6.5, similar to the normal pH of the skin, should be selected.”13
The Bottom Line
When buying a cleanser to use on your face or other parts of your body that tend to break out, it is safest to look for a liquid cleanser made specifically for the face. Look for words like “ultra-gentle” or “for sensitive skin” and you are likely to be purchasing a cleanser with a slightly acidic pH that will be safe to use on acne-prone areas of the body.
Avoid soap entirely.
References
- Lim, K. F. Negative pH does exist. J Chem Ed 83, 1465 (2006). https://pubs.acs.org/doi/abs/10.1021/ed083p1465
- Zlotogorski, A. Distribution of skin surface pH on the forehead and cheek of adults. Arch Dermatol Res 279, 398 – 401 (1987). https://www.ncbi.nlm.nih.gov/pubmed/3674963
- Levin, J. & Miller, R. A Guide to the Ingredients and Potential Benefits of Over-the-Counter Cleansers and Moisturizers for Rosacea Patients. J Clin Aesthet Dermatol 4, 31 – 49 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21909456
- Hachem, J. P. et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Investig Dermatol 121, 345 – 53 (2003). https://www.ncbi.nlm.nih.gov/pubmed/12880427
- Levin, J. & Maibach, H. Human skin buffering capacity: An overview. Skin Res Technol 14, 121 – 126 (2008). https://www.ncbi.nlm.nih.gov/pubmed/18412552
- Mukhopadhyay, P. Cleansers and their role in various dermatological disorders. Indian J Dermatol 56, 2 – 6 (2011). https://www.ncbi.nlm.nih.gov/pubmed/21572782
- Korting, H. C. & Braun-Falco, O. The effect of detergents on skin pH and its consequences. Clin Dermatol 14, 23-27 (1996). https://www.ncbi.nlm.nih.gov/pubmed/8901395
- Ananthapadmanabhan, K. P. et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther 17 Suppl 1, 16 – 25 (2004). https://www.ncbi.nlm.nih.gov/pubmed/14728695
- Korting, H. C. & Ponce-Pöschl, E., Klövekorn, W., Schmötzer, G., Arens-Corell, M. & Braun-Falco O. The influence of the regular use of a soap or an acidic syndet bar on pre-acne. Infection 23, 89-93 (1995). https://www.ncbi.nlm.nih.gov/pubmed/7622270
- Korting, H. C. et al. Influence of topical erythromycin preparations for acne vulgaris on skin surface pH. Clin Investig 71, 644 – 648 (1993). https://www.ncbi.nlm.nih.gov/pubmed/8219662
- Takagi, Y. et al. The long-term use of soap does not affect the pH-maintenance mechanism of human skin. Skin Res Technol 21, 144 – 148 (2015). https://www.ncbi.nlm.nih.gov/pubmed/25073884
- Duncan, C. N. et al. The effect of an acidic cleanser versus soap on the skin pH and micro-flora of adult patients: A non-randomised two group crossover study in an intensive care unit. Intensive Crit Care Nurs 29, 291 – 296 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23665029
- Ali, S. M. & Yosipovitch, G. Skin pH: from basic science to basic skin care. Acta Derm Venereol 93, 261-267 (2013). https://www.ncbi.nlm.nih.gov/pubmed/23322028